
The Impact of Stress on Periodontal Health: A Biomarker-Based Review of Current Evidence
Dentistry受け取った 02 Feb 2025 受け入れられた 24 Feb 2025 オンラインで公開された 25 Feb 2025
ISSN: 2995-8067 | Quick Google Scholar
Next Full Text
In Biological Research, Single-cell RNA Sequencing Answers the Puzzles around Unusual Cell Types


受け取った 02 Feb 2025 受け入れられた 24 Feb 2025 オンラインで公開された 25 Feb 2025
Numerous studies indicate that periodontal diseases affect a large portion of the adult population, and gum disease is a primary cause of losing teeth in the future. Periodontal diseases are a global health concern. Periodontal diseases are the most common and serious oral illnesses, affecting not only the gums but also the tissue and bone that support the teeth. It begins as gingivitis, a slight swelling of the gums resulting from the buildup of bacterial plaque. If not treated appropriately, it may advance to periodontitis, where the inflammation and infection spread deeper, causing damage to the tissue and bone. The scientific data that deals with stress is vast; it covers different theories of the influence of stress, the consequences of stress on the body and the brain, and the other contributors of stress. The scientific data contains heterogeneous findings about stress's causes, nature, and subsequent effects.
Aim: Since stress is a prevalent problem in modern life, the present work aims to estimate the association between stress and periodontal diseases, investigating the central relations highlighted in the scientific data.
Methods: The search comprised electronic databases: MEDLINE (PubMed), and Google Scholar, open access journals published by Elsevier. The keywords for the search were "stress," "periodontal diseases," "cortisol," and "saliva" in different combinations. The search considered works published from 2014 until December 2024. The procedure is shown in the PRISMA flowchart.
Conclusion: This review highlights the links between physiological stress, the stress hormone cortisol, and periodontal diseases, emphasizing the importance of considering stress as a risk factor in oral health. Understanding these relationships can help clarify discrepancies in treatment effectiveness and inform future healthcare practices.
Gum diseases encompass various inflammatory conditions that impact the structures that support teeth, such as the gums, bone, and periodontal ligament. These conditions can result in tooth loss and may also contribute to systemic inflammation. The significant occurrence of periodontal disease in teenagers, adults, and seniors poses a public health issue []. In line with the World Health Organization (WHO), gum disease is a major contributor to tooth loss. It affects about 40% of people over 30 years old in the United States and nearly 60% of individuals over 65 years old [].
Inflammatory periodontal diseases are prevalent chronic disorders that have multiple contributing factors. The accumulation of plaque and the imbalance between the microbial factors and the host's immune response are crucial in their progression [].
The main factor that leads to periodontal diseases is the combined presence of various bacteria colonizing the oral tissues. Dental plaque biofilm is regarded as a primary cause of periodontal diseases. Microbial enzymes and toxins, rapid bacterial proliferation, and localized phagocytic reactions contribute to the pathology of the odonto-gingival junction, chronic inflammation, periodontium destruction, and periodontal pocket formation [].
The research of periodopathogens and inflammation has obtained interest from researchers beyond the field of dentistry due to the potential impact of periodontitis on the onset and advancement of several systemic diseases []. Periodontal bacteria and their metabolic by-products in the oral cavity can influence the immune response outside of the mouth, potentially leading to systemic diseases [].
Historically, periodontitis was viewed as an inflammatory disease affecting only the periodontal tissues in the mouth. The scientific community has grown awareness of the associations between periodontal disease and various other chronic systemic diseases and conditions [,].
Bacterial biofilm is the main factor responsible for periodontal diseases in those prone to them. In addition to bacterial plaque, various environmental factors and personal characteristics may increase the risk of periodontal disease or alter its progression.
Due to the widespread nature of stress, this study seeks to assess the relationship between stress and periodontal diseases by exploring the key connections emphasized in existing research.
A narrative style of review was chosen to prepare this research paper. An electronic literature search was done across such databases, as Google Scholar, and MEDLINE (PubMed), and in open-access publications that are released by Elsevier, using MESH terms such as "stress," "periodontal diseases," "cortisol," and "saliva" in different combinations. Current reports in the literature concerning the interconnection of psychoemotional stress and periodontal diseases of human adults are considered. The selected articles were peer-reviewed and written in English, and relevant studies were distributed equally between coauthors for critical analysis before being included in this review. The authors follow the principles of the PRISMA flow chart to prepare this narrative review. The search considered works published from 2014 until December 2024 using the abovementioned keywords, as we want to include only up-to-date information. The inclusion criteria are as follows: (i) full-text journal articles written in English; (ii) books and book chapters written in English. The exclusion criteria are as follows: (i) case reports (clinical trials), (ii) conference papers, (iii) materials published earlier than 2014, (iv) randomized controlled studies, and (v) editorials.
1) The search was carried out in MEDLINE (PubMed), Google Scholar, and open-access publications that are released by Elsevier, using the keywords “stress”, “cortisol”, “saliva” and “periodontal diseases” in various combinations. In total, 2891 records were found. After the removal of duplicates, 1448 articles were left and screened by title and abstract.
2) Co-authors analyzed 1443 records for compliance with the inclusion and exclusion criteria. Scientific papers were distributed equally among the authors for screening. Additional documents (4) published before 2014 were identified by hand-searching the reference lists and included in the manuscript due to the importance of the information discussed. In total, 1349 records were deleted, i.e., 94 records remained reviewed in full text.
3) All selected records were distributed among all authors for reading the full-text articles and preparing the manuscript. At this stage, fifty-nine (59) records were excluded after reviewing the full text for eligibility. The procedure is shown in Figure 1 in the PRISMA flowchart.
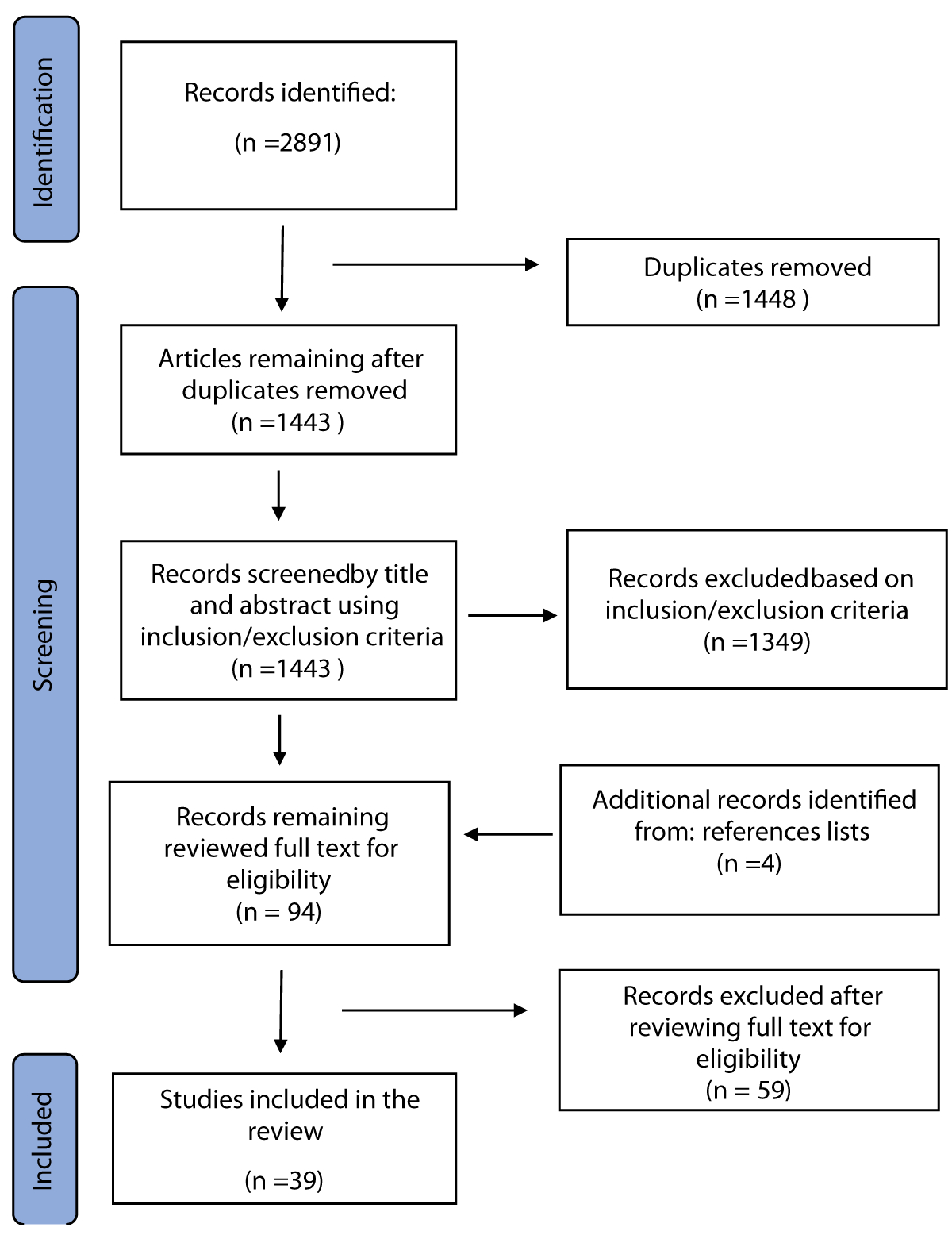 Figure 1: PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram of inclusion/exclusion criteria.
Figure 1: PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram of inclusion/exclusion criteria.Diverse forms of periodontal diseases include such influencing factors: local (i.e., because of plaque accumulation and oral hygiene habits), systemic, behavioral, and environmental factors (e.g., emotional stress and smoking habits) (Figure 2) [].
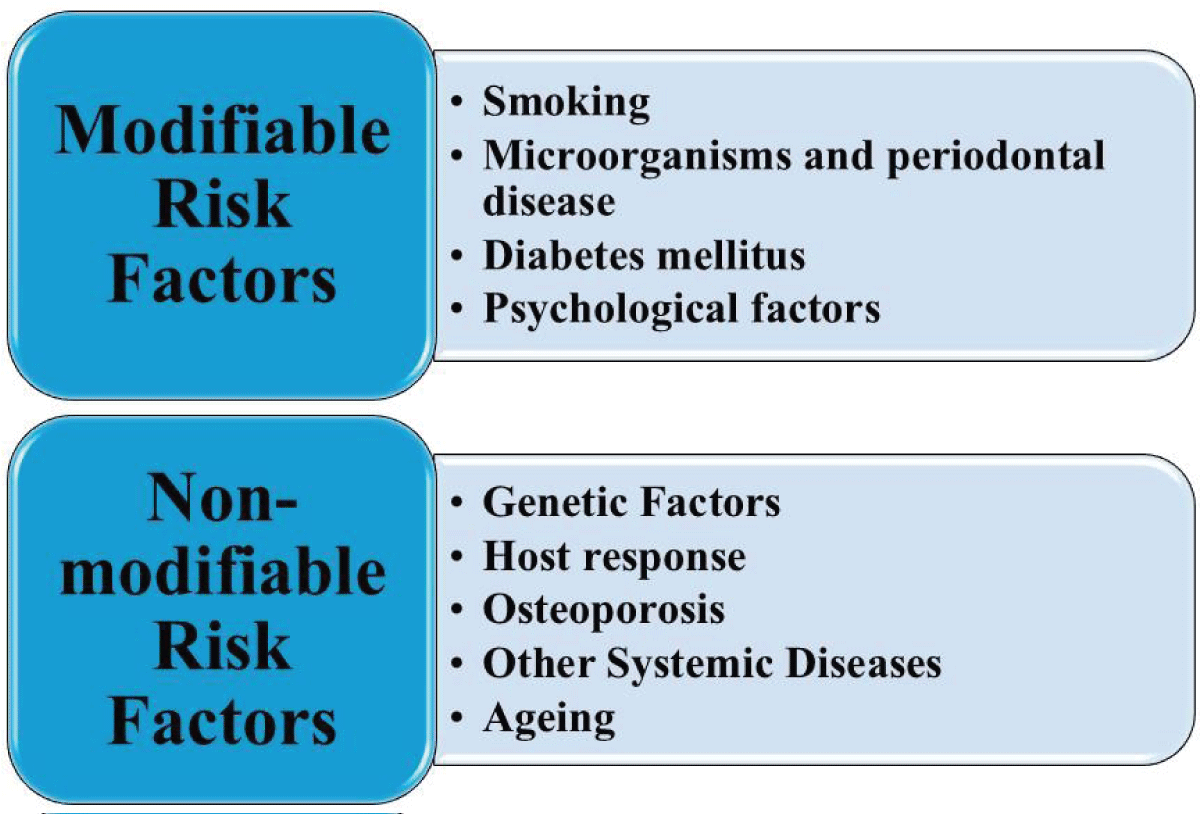 Figure 2: Risk factors for periodontitis. (Adapted from existing literature and created by authors using MS PowerPoint).
Figure 2: Risk factors for periodontitis. (Adapted from existing literature and created by authors using MS PowerPoint).Psychological stress affects the immune system, causing inflammatory responses [,]. Such type of stress may contribute to chronic inflammatory diseases, such as periodontitis []. Scientific research has evidenced the relationship between inflammatory disease and psychological stress, and several studies have evaluated the connections between neuroendocrine responses and psychological factors in terms of the severity and level of periodontal diseases [,]. Current research has not yet demonstrated a direct connection between periodontal diseases, psychological stress, and alterations in the endocrine system related to stress, and the HPA axis.
‘Stress’ is the word that is known to everyone. Stress is merely a reaction to physical and emotional pressures. Groundbreaking physician and endocrinologist Hans Selye, who is recognized in various fields as the originator of stress theory, characterized stress as “the state manifested by a specific syndrome which consists of all the nonspecifically-induced changes within a biologic system” [,].
Stress can be classified into different types depending on its duration, origin, and the way individuals respond [] (Figure 3).
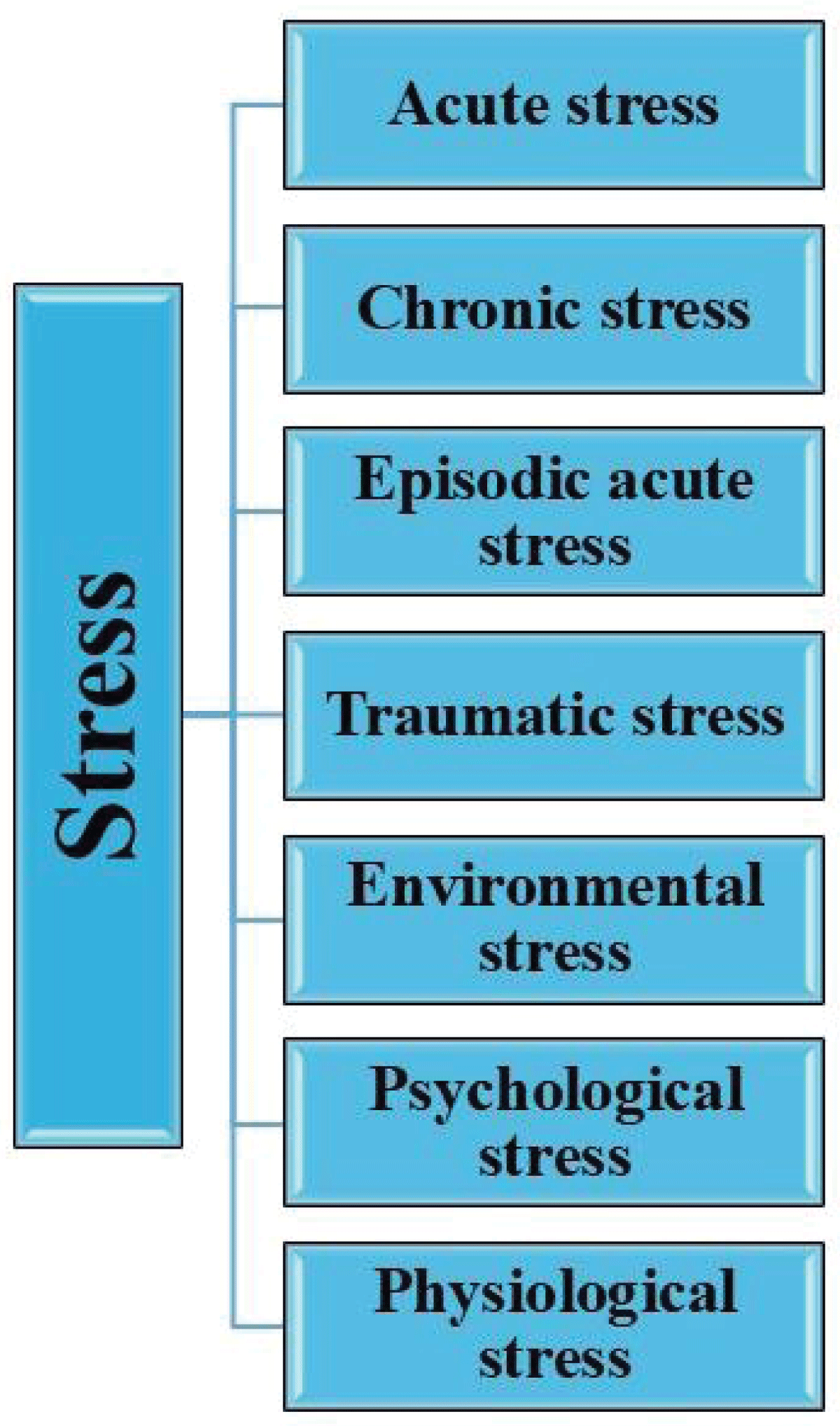 Figure 3: Types of stress. (Adapted from existing literature and created by authors using MS PowerPoint).
Figure 3: Types of stress. (Adapted from existing literature and created by authors using MS PowerPoint).A complicated interaction between the nervous, endocrine, and immune systems facilitates a stress response, which activates the sympathetic-adreno-medullary (SAM) axis, the hypothalamic-pituitary-adrenal (HPA) axis, and the immune response (Figures 4,5) [,].
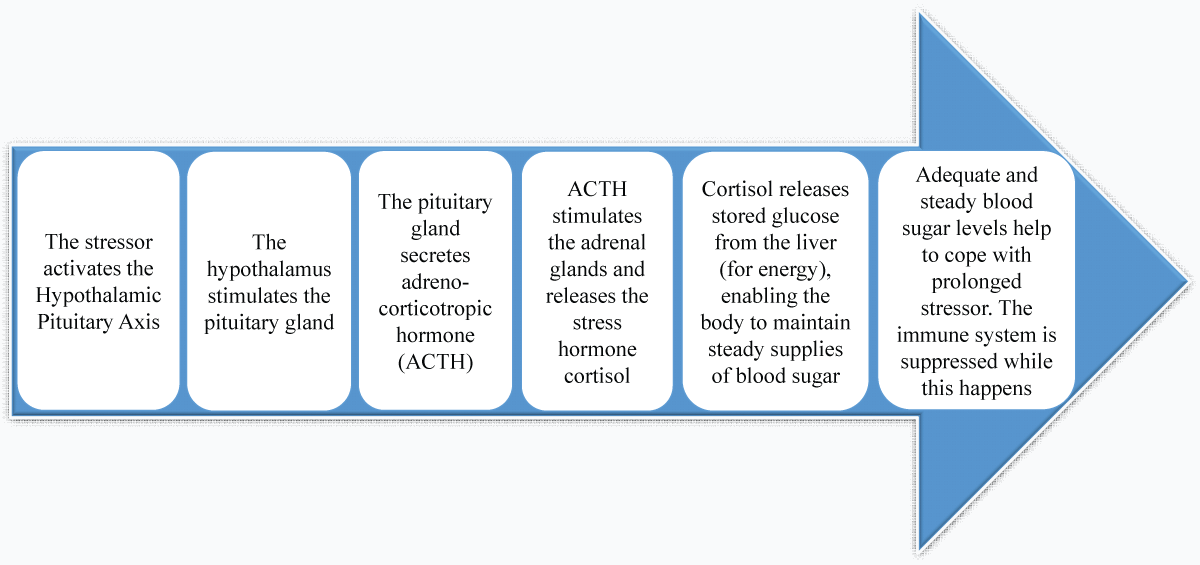 Figure 4: Long-term stress response: hypothalamic-pituitary-adrenal axis (HPA). (Adapted from existing literature and created by authors using MS PowerPoint).
Figure 4: Long-term stress response: hypothalamic-pituitary-adrenal axis (HPA). (Adapted from existing literature and created by authors using MS PowerPoint).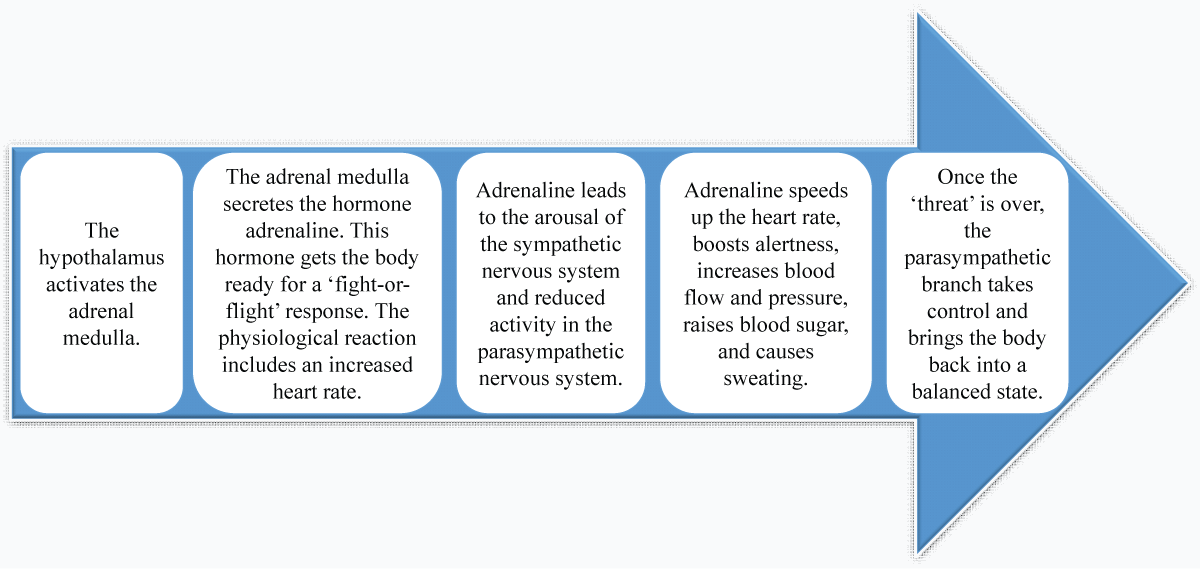 Figure 5: Sympathomedullary Pathway (SAM). (Adapted from existing literature and created by authors using MS PowerPoint).
Figure 5: Sympathomedullary Pathway (SAM). (Adapted from existing literature and created by authors using MS PowerPoint).Stress has an unmediated influence on periodontal health due to changes in behavior and lifestyle. It leads to increased smoking and alcohol use, unhealthy eating habits, neglect of oral hygiene, and poor adherence to dental care practices []. Such behavior affects periodontal health through a direct biological impact, mediated by alterations in saliva, variations in gingival blood flow, and the host's immune response regulation [].
Different testing methods are employed to evaluate the human stress response, mainly through biological markers []. Measuring the levels of stress hormones like cortisol, epinephrine, and norepinephrine in blood, saliva, and urine offers objective measures of the body's physiological reaction to stress. These markers indicate the functioning of the HPA axis and the SAM system.
Glucocorticoids are the main hormones involved in the stress response and oversee various physiological functions. Additionally, synthetic versions of these compounds are commonly utilized in medical settings to treat inflammatory conditions and autoimmune disorders [].
Saliva is a perfect biological fluid for diagnostic purposes since it remains fluid and does not clot. It maintains stability for 24 hours at room temperature and one week at 4 °C. A significant reason for the extensive application of saliva sampling is its convenience, as it is an easy-to-handle, non-invasive method. Utilizing saliva for medical diagnostics is cost-effective, non-invasive, painless, and convenient for various patient demographics (Figure 6).
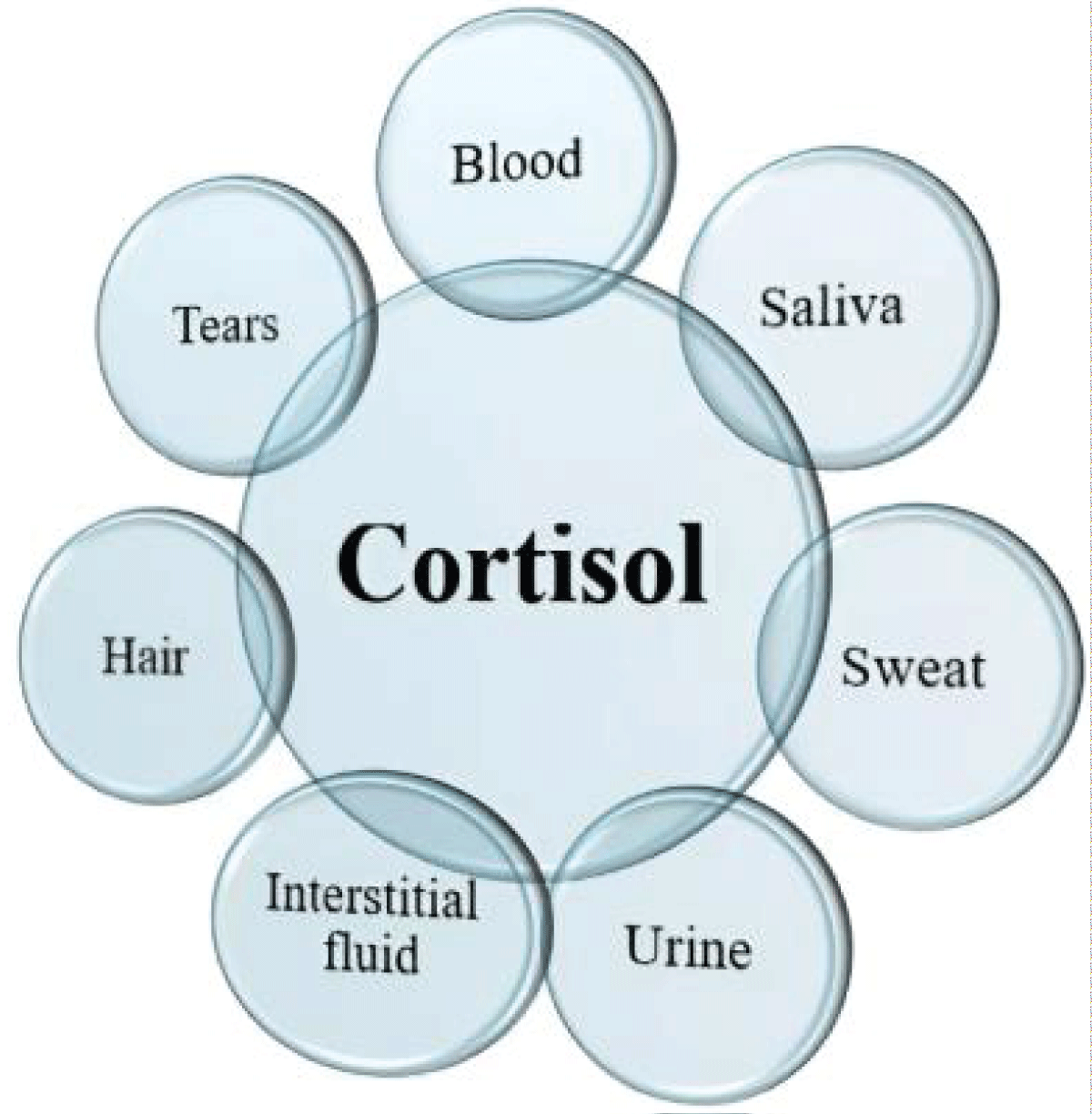 Figure 6: Various biological sources of cortisol. (Adapted from existing literature and created by authors using MS PowerPoint).
Figure 6: Various biological sources of cortisol. (Adapted from existing literature and created by authors using MS PowerPoint).Cortisol is a steroid hormone secreted by the adrenal cortex and is produced through the Hypothalamic-Pituitary-Adrenal (HPA) axis []. The signaling pathway begins in the hypothalamus, which gets neurological inputs from the Para-Ventricular Nucleus (PVN) and the suprachiasmatic nucleus of the hypothalamus. In reaction to specific triggers, cells in the PVN release Corticotropin-releasing Factor (CRF), which encourages the pituitary gland to secrete Adrenocorticotropic Hormone (ACTH) which enters the bloodstream, causing a steroid-producing effect and prompting the adrenal cortex to secrete cortisol.
Cortisol, the main glucocorticoid in humans, is secreted when the HPA axis is stimulated by psychological stress. This release process increases glucose levels through gluconeogenesis and raises blood pressure []. Long-term stress can lead to the dysregulation of the HPA axis that induces abnormal basal and stress levels of HPA hormones, including cortisol and DHEA [].
Cortisol is a powerful anti-inflammatory hormone that helps mobilize glucose for energy and manages inflammation []. Extended or intensified stress responses can cause cortisol dysregulation leading to extensive inflammation and discomfort []. Scientific research compared patients with chronic periodontitis with and without stress; interestingly, increased salivary cortisol levels were found for those with stress.
Cortisol levels typically peak in the morning, around 30-50 minutes after waking up, and gradually decline throughout the day, following a circadian pattern, with the lowest levels occurring at night [].
DHEA is a corticosteroid produced by the adrenal glands in reaction to the activation of the HPA axis, and it is also found in the gonads and central nervous system. Dehydroepiandrosterone is particularly interesting. The sulfated form of DHEA (DHEAS) is man's most abundant circulating steroid []. Blood DHEA and DHEAS increase markedly during early childhood and again during puberty. Physical ill-health or more acute events result in an elevated cortisol level but lowered DHEA and altered cortisol/DHEA ratios. A critical feature of DHEA(S) is thus that it can change independently of cortisol during everyday life and in illness.
DHEA is a biomarker that typically takes about one hour to elevate in response to acute stress, and its levels rise following acute psychological stress, regardless of the kind and duration of stress []. Nevertheless, there have been few studies concerning salivary DHEA in the field of dentistry.
Chromogranin A (GRN-A, also known as secretory protein I) is a glycoprotein that is released alongside catecholamines in the adrenal medulla and sympathetic nerve endings []. The release is regulated by the precise interaction between the HPA axis and the sympathetic nervous system. Thus, for assessing psychological stress this glycoprotein is identified as a novel biomarker for stress.
Chromogranin A is an acidic glycoprotein, phosphorylated, secreted, and stored; it is released simultaneously as catecholamines from the adrenal medulla. Secretory protein I is also found in sympathetic nerve endings and the ductal cells of the human submandibular gland, where it is stored and released. It has been demonstrated that Chromogranin A leads to the formation of several bioactive peptides, including catestatin, which may contribute to inflammation [-].
The alpha-amylase enzyme is a crucial component of saliva, comprising 40% to 50% of the overall salivary proteins, and most of it is secreted from the parotid gland. SAA plays a vital role in carbohydrate hydrolysis. Furthermore, alpha-amylase may contribute to the oral immune system [].
Anxiety activates the Sympathetic Nervous System (SNS) and the Hypothalamic-pituitary-adrenal axis (HPA). As a result of this process, SNS is activated, and the release of epinephrine and norepinephrine is initiated from the adrenal center. Finally, norepinephrine increases the secretion of SAA from parotid and submandibular acinar cells.
SAA measurement is more straightforward, non-invasive, stress-free, and has a high potential for repeatability when contrasted with serum catecholamine measurement [,]. Salivary alpha-amylase has been found to be a reliable marker reflecting sympathetic nervous system activity during stress. It also displays antimicrobial activity [].
The connection between periodontitis and psycho-neuro-immunological factors, including psychological stress, cortisol, Dehydroepiandrosterone (DHEA), and chromogranin A, has not been extensively researched.
The relationship between depression, Сhronic Periodontitis (CP), and Salivary Cortisol Level (SCL) was evaluated in a cross-sectional study conducted by by Refulio, et al. []. A significant association between SCL and the presence of CP was found. This result indicates that psychoneuroimmunologic elements could be involved in the onset of periodontal diseases, which aligns with earlier findings [].
The findings from Lee YH, et al. [] indicated that psychological stress activates the HPA axis, which is a neuroendocrine system related to stress, in both patients with periodontal diseases and healthy individuals. Moreover, the progression of periodontal diseases promotes the rise of salivary cortisol and chromogranin A levels. In the group of periodontitis, the cortisol/DHEA ratio and DHEA levels were elevated compared to the healthy control group.
The research conducted by Obulareddy VT, et al. [] found that cortisol in saliva was present even in clinically healthy individuals. However, it was relatively minimal compared to the diseased groups. Such a condition may arise because the healthy oral environment contains different types of bacteria that can cause a mild inflammatory response in the periodontal tissues, indicating that cortisol in clinically healthy tissues may facilitate controlled chemotaxis, which is essential for immune regulation.
The research conducted by Rahate, et al. and Zhang, et al. [,] focused on analyzing the serum and saliva concentrations of ghrelin and cortisol along with the levels of IL-1B in patients with periodontitis, both smokers and non-smokers. Research on clinical parameters indicates that in patients with periodontitis, stress and smoking habits contribute to a greater level of tissue destruction.
Recent research indicates a favourable link between stress and periodontitis, suggesting that stress may act as a risk factor for this condition []. Scientific research has shown the role of stress hormones on the growth of selected periodontitis-related bacteria. These findings underscore the significance of stress about periodontal health and the nitrite levels in saliva, and they emphasize a possible distinct influence of certain bacteria on saliva's nitrite levels [].
The association between cortisol and chronic periodontitis may be linked to the suppressive influence of the HPA axis on the inflammatory response because all of the immune response components are inhibited by cortisol [].
The results of this research should be considered alongside certain limitations. First, not all significant articles are available in the journals that are indexed in the databases utilized for the search. The duration required for an article to be reviewed and then indexed in these databases is quite lengthy (the minimum time needed is approximately two months, while the maximum time is indefinite, but it could extend to a year or even longer), resulting in the exclusion of some articles from our review. It is also possible that the significant articles do not contain the keywords we were targeting. A restricted search period (10 years) might leave out valuable study manuscripts from our review criteria.
Investigating specific biomarkers related to stress, such as cortisol levels, alpha-amylase, and C-reactive protein in the context of periodontal diseases will be able to clarify the biochemical pathways through which stress affects gum health.
Future research should investigate the relationship between periodontal diseases and stress, with a focus on potential prevention strategies.
A current review of existing research in the field has summarized the relationship between stress and periodontal health. This may help clarify the discrepancies and build a more substantial evidence base, potentially shaping future healthcare practices.
Based on the findings from this review, oral health practitioners need to consider stress factors as part of the risk factors for periodontal diseases, their severity, and the reduced effectiveness of treatments, especially for overall health reasons.
Every patient who has stress, emotional depression, and a level of anxiety condition requires more care and perhaps more continuous supportive therapy to reduce the risk of periodontitis.
Monitoring psychological stress and related biomarkers is vital in providing insight into cause-and-effect relationships.
SB: Conceptualization, Investigation, Writing-original draft, Writing-review & editing, Supervision.
ML: Conceptualization, Investigation, Validation, Writing-original draft, Writing-review & editing.
PO: Investigation Writing-original draft, Writing-review & editing,
All authors read and approved the submitted version.
Bertolini M, Clark D. Periodontal disease as a model to study chronic inflammation in aging. Geroscience. 2024 Aug;46(4):3695-3709. doi: 10.1007/s11357-023-00835-0. Epub 2023 Jun 7. PMID: 37285008; PMCID: PMC11226587.
Eke PI, Wei L, Borgnakke WS, Thornton-Evans G, Zhang X, Lu H, McGuire LC, Genco RJ. Periodontitis prevalence in adults ≥ 65 years of age, in the USA. Periodontol 2000. 2016 Oct;72(1):76-95. doi: 10.1111/prd.12145. PMID: 27501492; PMCID: PMC8223257.
Könönen E, Gursoy M, Gursoy UK. Periodontitis: A Multifaceted Disease of Tooth-Supporting Tissues. J Clin Med. 2019 Jul 31;8(8):1135. doi: 10.3390/jcm8081135. PMID: 31370168; PMCID: PMC6723779.
Kotb SH. Overview of Periodontal Disease, Etiology, Pathogenesis, Diagnosis and Treatment Therapy.
Denefil O, Chorniy S, Boitsaniuk S, Manashchuk N, Chornij N, Levkiv M, Tverdokhlib N, Loza K. Analysis of microbiocenosis of a gingival sulcus and periodontal pockets of patients with periodontal diseases associated with systemic pathology. Exploration of Medicine. 2023 Dec 11;4(6):942-55.
Denefil O, Chorniy S, Boitsaniuk S, Chornij N, Levkiv M, Patskan L, Pohoretska K, Manashchuk N, Zaliznyak M, Tverdokhlib N. Comparative analysis of dysbiotic changes in the oral cavity of patients with periodontal diseases and systemic pathologies. Exploration of Medicine. 2024 Sep 3;5(5):574-83.
Villoria GEM, Fischer RG, Tinoco EMB, Meyle J, Loos BG. Periodontal disease: A systemic condition. Periodontol 2000. 2024 Oct;96(1):7-19. doi: 10.1111/prd.12616. Epub 2024 Nov 4. PMID: 39494478; PMCID: PMC11579822.
Martínez-García M, Hernández-Lemus E. Periodontal Inflammation and Systemic Diseases: An Overview. Front Physiol. 2021 Oct 27;12:709438. doi: 10.3389/fphys.2021.709438. PMID: 34776994; PMCID: PMC8578868.
Nazir MA. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int J Health Sci (Qassim). 2017 Apr-Jun;11(2):72-80. PMID: 28539867; PMCID: PMC5426403.
Hahnefeld L, Hackel J, Trautmann S, Angioni C, Schreiber Y, Gurke R, Thomas D, Wicker S, Geisslinger G, Tegeder I. Healthy plasma lipidomic signatures depend on sex, age, body mass index, and contraceptives but not perceived stress. Am J Physiol Cell Physiol. 2024 Dec 1;327(6):C1462-C1480. doi: 10.1152/ajpcell.00630.2024. Epub 2024 Oct 22. PMID: 39437447.
Lee YH, Suk C, Shin SI, Hong JY. Salivary cortisol, dehydroepiandrosterone, and chromogranin A levels in patients with gingivitis and periodontitis and a novel biomarker for psychological stress. Front Endocrinol (Lausanne). 2023 Apr 11;14:1147739. doi: 10.3389/fendo.2023.1147739. PMID: 37113482; PMCID: PMC10126469.
Decker AM, Kapila YL, Wang HL. The psychobiological links between chronic stress-related diseases, periodontal/peri-implant diseases, and wound healing. Periodontol 2000. 2021 Oct;87(1):94-106. doi: 10.1111/prd.12381. PMID: 34463997; PMCID: PMC8459609.
Hilgert JB, Hugo FN, Bandeira DR, Bozzetti MC. Stress, cortisol, and periodontitis in a population aged 50 years and over. J Dent Res. 2006 Apr;85(4):324-8. doi: 10.1177/154405910608500408. PMID: 16567552.
James KA, Stromin JI, Steenkamp N, Combrinck MI. Understanding the relationships between physiological and psychosocial stress, cortisol and cognition. Front Endocrinol (Lausanne). 2023 Mar 6;14:1085950. doi: 10.3389/fendo.2023.1085950. PMID: 36950689; PMCID: PMC10025564.
Oosenbrug E. Hans Selye: Salesman of Stress. York University; 2011 Dec.
Lushchak VI. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem Biol Interact. 2014 Dec 5;224:164-75. doi: 10.1016/j.cbi.2014.10.016. Epub 2014 Oct 28. PMID: 25452175.
Mifsud KR, Reul JMHM. Mineralocorticoid and glucocorticoid receptor-mediated control of genomic responses to stress in the brain. Stress. 2018 Sep;21(5):389-402. doi: 10.1080/10253890.2018.1456526. Epub 2018 Apr 4. PMID: 29614900.
Chu B, Marwaha K, Sanvictores T, Awosika AO, Ayers D. Physiology, stress reaction. InStatPearls [Internet] 2024 May 7. StatPearls Publishing.
Alhumaidan AA, Al-Aali KA, Vohra F, Javed F, Abduljabbar T. Comparison of Whole Salivary Cortisol and Interleukin 1-Beta Levels in Light Cigarette-Smokers and Users of Electronic Nicotine Delivery Systems before and after Non-Surgical Periodontal Therapy. Int J Environ Res Public Health. 2022 Sep 8;19(18):11290. doi: 10.3390/ijerph191811290. PMID: 36141565; PMCID: PMC9517181.
Corridore D, Saccucci M, Zumbo G, Fontana E, Lamazza L, Stamegna C, Di Carlo G, Vozza I, Guerra F. Impact of Stress on periodontal health: literature revision. InHealthcare 2023 May 22. 11:1516.
Develioglu H, Korkmaz S, Dundar S, Schlagenhauf U. Investigation of the levels of different salivary stress markers in chronic periodontitis patients. J Oral Biol Craniofac Res. 2020 Oct-Dec;10(4):514-518. doi: 10.1016/j.jobcr.2020.07.020. Epub 2020 Aug 12. PMID: 32874881; PMCID: PMC7452230.
Ramamoorthy S, Cidlowski JA. Corticosteroids: Mechanisms of Action in Health and Disease. Rheum Dis Clin North Am. 2016 Feb;42(1):15-31, vii. doi: 10.1016/j.rdc.2015.08.002. PMID: 26611548; PMCID: PMC4662771.
Andrea Scribante, Matteo Pellegrini, Martina Ghizzoni, Federica Pulicari, Aldo Bruno Giannм, Francesco Spadari. "Exploring the Potential Clinical Applications of Salivary Cortisol in the Diagnosis and Management of Cushing’s Syndrome, Diabetes, Depression, and Periodontal Disease: A Systematic Review", The Open Dentistry Journal, 2024.
Pan X, Wang Z, Wu X, Wen SW, Liu A. Salivary cortisol in post-traumatic stress disorder: a systematic review and meta-analysis. BMC Psychiatry. 2018 Oct 5;18(1):324. doi: 10.1186/s12888-018-1910-9. PMID: 30290789; PMCID: PMC6173866.
Basson R, O'Loughlin JI, Weinberg J, Young AH, Bodnar T, Brotto LA. Dehydroepiandrosterone and cortisol as markers of HPA axis dysregulation in women with low sexual desire. Psychoneuroendocrinology. 2019 Jun;104:259-268. doi: 10.1016/j.psyneuen.2019.03.001. Epub 2019 Mar 8. PMID: 30909007; PMCID: PMC7343293.
Hannibal KE, Bishop MD. Chronic stress, cortisol dysfunction, and pain: a psychoneuroendocrine rationale for stress management in pain rehabilitation. Phys Ther. 2014 Dec;94(12):1816-25. doi: 10.2522/ptj.20130597. Epub 2014 Jul 17. PMID: 25035267; PMCID: PMC4263906.
Lopez-Jornet P, Zavattaro E, Mozaffari HR, Ramezani M, Sadeghi M. Evaluation of the Salivary Level of Cortisol in Patients with Oral Lichen Planus: A Meta-Analysis. Medicina (Kaunas). 2019 May 27;55(5):213. doi: 10.3390/medicina55050213. PMID: 31137861; PMCID: PMC6571959.
Vandana S, Kavitha B, Sivapathasundharam B. Salivary cortisol and dehydroepiandrosterone as oral biomarkers to determine stress in patients with recurrent aphthous stomatitis. J Oral Maxillofac Pathol. 2019 May-Aug;23(2):213-217. doi: 10.4103/jomfp.JOMFP_282_18. PMID: 31516226; PMCID: PMC6714283.
Lennartsson AK, Sjörs A, Jonsdottir IH. Indication of attenuated DHEA-s response during acute psychosocial stress in patients with clinical burnout. J Psychosom Res. 2015 Aug;79(2):107-11. doi: 10.1016/j.jpsychores.2015.05.011. Epub 2015 May 21. PMID: 26071787.
Tananska VT. Salivary α-Amylase And Chromogranin A In Anxiety-Related Research. Folia Med (Plovdiv). 2014 Oct-Dec;56(4):233-6. doi: 10.1515/folmed-2015-0001. PMID: 26444351.
Mizuhashi F, Koide K, Toya S, Takahashi M, Mizuhashi R, Shimomura H. Levels of the antimicrobial proteins lactoferrin and chromogranin in the saliva of individuals with oral dryness. J Prosthet Dent. 2015 Jan;113(1):35-8. doi: 10.1016/j.prosdent.2013.12.028. Epub 2014 Oct 7. PMID: 25300178.
Saruta J, Tsukinoki K, Sasaguri K, Ishii H, Yasuda M, Osamura YR, Watanabe Y, Sato S. Expression and localization of chromogranin A gene and protein in human submandibular gland. Cells Tissues Organs. 2005;180(4):237-44. doi: 10.1159/000088939. PMID: 16330879.
Ball J, Darby I. Mental health and periodontal and peri-implant diseases. Periodontol 2000. 2022 Oct;90(1):106-124. doi: 10.1111/prd.12452. Epub 2022 Aug 1. PMID: 35913583; PMCID: PMC9804456.
Sahu GK, Upadhyay S, Panna SM. Salivary alpha amylase activity in human beings of different age groups subjected to psychological stress. Indian J Clin Biochem. 2014 Oct;29(4):485-90. doi: 10.1007/s12291-013-0388-y. Epub 2013 Oct 6. PMID: 25298630; PMCID: PMC4175699.
Jafari A, Pouramir M, Shirzad A, Motallebnejad M, Bijani A, Moudi S, Abolghasem-Zade F, Dastan Z. Evaluation of salivary alpha amylase as a biomarker for dental anxiety. Iranian Journal of Psychiatry and Behavioral Sciences. 2018 Mar 31;12(1).
Refulio Z, Rocafuerte M, de la Rosa M, Mendoza G, Chambrone L. Association among stress, salivary cortisol levels, and chronic periodontitis. J Periodontal Implant Sci. 2013 Apr;43(2):96-100. doi: 10.5051/jpis.2013.43.2.96. Epub 2013 Apr 30. PMID: 23678393; PMCID: PMC3651943.
Seizer L, Schubert C. On the Role of Psychoneuroimmunology in Oral Medicine. Int Dent J. 2022 Dec;72(6):765-772. doi: 10.1016/j.identj.2022.07.002. Epub 2022 Sep 30. PMID: 36184323; PMCID: PMC9676547.
Obulareddy VT, Chava VK, Nagarakanti S. Association of Stress, Salivary Cortisol, and Chronic Periodontitis: A Clinico-biochemical Study. Contemp Clin Dent. 2018 Sep;9(Suppl 2):S299-S304. doi: 10.4103/ccd.ccd_289_18. PMID: 30294161; PMCID: PMC6169263.
Rahate PS, Kolte RA, Kolte AP, Lathiya VN, Gupta M, Chari S. Evaluation of stress, serum, and salivary ghrelin and cortisol levels in smokers and non-smokers with Stage III periodontitis: A cross-sectional study. J Periodontol. 2022 Aug;93(8):1131-1140. doi: 10.1002/JPER.21-0373. Epub 2022 Jan 25. PMID: 34859428.
Zhang H, Chen B, Pan C, Zhang A. To evaluate the serum cortisol, salivary cortisol, and serum interleukin-1 B level in patients of chronic periodontitis with smoking and stress and without smoking and stress. Medicine (Baltimore). 2021 Aug 6;100(31):e26757. doi: 10.1097/MD.0000000000026757. PMID: 34397819; PMCID: PMC8341332.
Ponzio E, Dolcini J, Sparabombe S, Firmani G, D'Errico MM, Barbadoro P. Psychological Stress and its relationship to Periodontal flora and salivary Nitrite/Nitrate. Int Dent J. 2024 Aug;74(4):746-753. doi: 10.1016/j.identj.2024.02.003. Epub 2024 Mar 27. PMID: 38538383; PMCID: PMC11287180.
Mudrika S, Muthukumar S, Suresh R. Relationship between salivary levels of cortisol and dehydroepiandrosterone levels in saliva and chronic periodontitis. Journal of the International Clinical Dental Research Organization. 2014 Jul 1;6(2):92-7.
Boitsaniuk S, Levkiv M, Ostrovskyi P. The Impact of Stress on Periodontal Health: A Biomarker-Based Review of Current Evidence. IgMin Res. February 25, 2025; 3(2): 097-103. IgMin ID: igmin288; DOI:10.61927/igmin288; Available at: igmin.link/p288
次のリンクを共有した人は、このコンテンツを読むことができます:
1Dental Therapy Department, Dental Faculty, I. Horbachevsky Ternopil National Medical University, Ternopil, Ukraine
2Ternopil City Municipal Dental Clinic, Ternopil, Ukraine
Address Correspondence:
Mariana Levkiv, Dental Therapy Department, Dental Faculty, I. Horbachevsky Ternopil National Medical University, Ternopil 46003, Ukraine, Email: [email protected]
How to cite this article:
Boitsaniuk S, Levkiv M, Ostrovskyi P. The Impact of Stress on Periodontal Health: A Biomarker-Based Review of Current Evidence. IgMin Res. February 25, 2025; 3(2): 097-103. IgMin ID: igmin288; DOI:10.61927/igmin288; Available at: igmin.link/p288
Copyright: © 2025 Boitsaniuk S, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
 Figure 1: PRISMA (Preferred Reporting Items for Systematic R...
Figure 1: PRISMA (Preferred Reporting Items for Systematic R...
 Figure 2: Risk factors for periodontitis. (Adapted from exis...
Figure 2: Risk factors for periodontitis. (Adapted from exis...
 Figure 3: Types of stress. (Adapted from existing literature...
Figure 3: Types of stress. (Adapted from existing literature...
 Figure 4: Long-term stress response: hypothalamic-pituitary-...
Figure 4: Long-term stress response: hypothalamic-pituitary-...
 Figure 5: Sympathomedullary Pathway (SAM). (Adapted from exi...
Figure 5: Sympathomedullary Pathway (SAM). (Adapted from exi...
 Figure 6: Various biological sources of cortisol. (Adapted f...
Figure 6: Various biological sources of cortisol. (Adapted f...
Bertolini M, Clark D. Periodontal disease as a model to study chronic inflammation in aging. Geroscience. 2024 Aug;46(4):3695-3709. doi: 10.1007/s11357-023-00835-0. Epub 2023 Jun 7. PMID: 37285008; PMCID: PMC11226587.
Eke PI, Wei L, Borgnakke WS, Thornton-Evans G, Zhang X, Lu H, McGuire LC, Genco RJ. Periodontitis prevalence in adults ≥ 65 years of age, in the USA. Periodontol 2000. 2016 Oct;72(1):76-95. doi: 10.1111/prd.12145. PMID: 27501492; PMCID: PMC8223257.
Könönen E, Gursoy M, Gursoy UK. Periodontitis: A Multifaceted Disease of Tooth-Supporting Tissues. J Clin Med. 2019 Jul 31;8(8):1135. doi: 10.3390/jcm8081135. PMID: 31370168; PMCID: PMC6723779.
Kotb SH. Overview of Periodontal Disease, Etiology, Pathogenesis, Diagnosis and Treatment Therapy.
Denefil O, Chorniy S, Boitsaniuk S, Manashchuk N, Chornij N, Levkiv M, Tverdokhlib N, Loza K. Analysis of microbiocenosis of a gingival sulcus and periodontal pockets of patients with periodontal diseases associated with systemic pathology. Exploration of Medicine. 2023 Dec 11;4(6):942-55.
Denefil O, Chorniy S, Boitsaniuk S, Chornij N, Levkiv M, Patskan L, Pohoretska K, Manashchuk N, Zaliznyak M, Tverdokhlib N. Comparative analysis of dysbiotic changes in the oral cavity of patients with periodontal diseases and systemic pathologies. Exploration of Medicine. 2024 Sep 3;5(5):574-83.
Villoria GEM, Fischer RG, Tinoco EMB, Meyle J, Loos BG. Periodontal disease: A systemic condition. Periodontol 2000. 2024 Oct;96(1):7-19. doi: 10.1111/prd.12616. Epub 2024 Nov 4. PMID: 39494478; PMCID: PMC11579822.
Martínez-García M, Hernández-Lemus E. Periodontal Inflammation and Systemic Diseases: An Overview. Front Physiol. 2021 Oct 27;12:709438. doi: 10.3389/fphys.2021.709438. PMID: 34776994; PMCID: PMC8578868.
Nazir MA. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int J Health Sci (Qassim). 2017 Apr-Jun;11(2):72-80. PMID: 28539867; PMCID: PMC5426403.
Hahnefeld L, Hackel J, Trautmann S, Angioni C, Schreiber Y, Gurke R, Thomas D, Wicker S, Geisslinger G, Tegeder I. Healthy plasma lipidomic signatures depend on sex, age, body mass index, and contraceptives but not perceived stress. Am J Physiol Cell Physiol. 2024 Dec 1;327(6):C1462-C1480. doi: 10.1152/ajpcell.00630.2024. Epub 2024 Oct 22. PMID: 39437447.
Lee YH, Suk C, Shin SI, Hong JY. Salivary cortisol, dehydroepiandrosterone, and chromogranin A levels in patients with gingivitis and periodontitis and a novel biomarker for psychological stress. Front Endocrinol (Lausanne). 2023 Apr 11;14:1147739. doi: 10.3389/fendo.2023.1147739. PMID: 37113482; PMCID: PMC10126469.
Decker AM, Kapila YL, Wang HL. The psychobiological links between chronic stress-related diseases, periodontal/peri-implant diseases, and wound healing. Periodontol 2000. 2021 Oct;87(1):94-106. doi: 10.1111/prd.12381. PMID: 34463997; PMCID: PMC8459609.
Hilgert JB, Hugo FN, Bandeira DR, Bozzetti MC. Stress, cortisol, and periodontitis in a population aged 50 years and over. J Dent Res. 2006 Apr;85(4):324-8. doi: 10.1177/154405910608500408. PMID: 16567552.
James KA, Stromin JI, Steenkamp N, Combrinck MI. Understanding the relationships between physiological and psychosocial stress, cortisol and cognition. Front Endocrinol (Lausanne). 2023 Mar 6;14:1085950. doi: 10.3389/fendo.2023.1085950. PMID: 36950689; PMCID: PMC10025564.
Oosenbrug E. Hans Selye: Salesman of Stress. York University; 2011 Dec.
Lushchak VI. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem Biol Interact. 2014 Dec 5;224:164-75. doi: 10.1016/j.cbi.2014.10.016. Epub 2014 Oct 28. PMID: 25452175.
Mifsud KR, Reul JMHM. Mineralocorticoid and glucocorticoid receptor-mediated control of genomic responses to stress in the brain. Stress. 2018 Sep;21(5):389-402. doi: 10.1080/10253890.2018.1456526. Epub 2018 Apr 4. PMID: 29614900.
Chu B, Marwaha K, Sanvictores T, Awosika AO, Ayers D. Physiology, stress reaction. InStatPearls [Internet] 2024 May 7. StatPearls Publishing.
Alhumaidan AA, Al-Aali KA, Vohra F, Javed F, Abduljabbar T. Comparison of Whole Salivary Cortisol and Interleukin 1-Beta Levels in Light Cigarette-Smokers and Users of Electronic Nicotine Delivery Systems before and after Non-Surgical Periodontal Therapy. Int J Environ Res Public Health. 2022 Sep 8;19(18):11290. doi: 10.3390/ijerph191811290. PMID: 36141565; PMCID: PMC9517181.
Corridore D, Saccucci M, Zumbo G, Fontana E, Lamazza L, Stamegna C, Di Carlo G, Vozza I, Guerra F. Impact of Stress on periodontal health: literature revision. InHealthcare 2023 May 22. 11:1516.
Develioglu H, Korkmaz S, Dundar S, Schlagenhauf U. Investigation of the levels of different salivary stress markers in chronic periodontitis patients. J Oral Biol Craniofac Res. 2020 Oct-Dec;10(4):514-518. doi: 10.1016/j.jobcr.2020.07.020. Epub 2020 Aug 12. PMID: 32874881; PMCID: PMC7452230.
Ramamoorthy S, Cidlowski JA. Corticosteroids: Mechanisms of Action in Health and Disease. Rheum Dis Clin North Am. 2016 Feb;42(1):15-31, vii. doi: 10.1016/j.rdc.2015.08.002. PMID: 26611548; PMCID: PMC4662771.
Andrea Scribante, Matteo Pellegrini, Martina Ghizzoni, Federica Pulicari, Aldo Bruno Giannм, Francesco Spadari. "Exploring the Potential Clinical Applications of Salivary Cortisol in the Diagnosis and Management of Cushing’s Syndrome, Diabetes, Depression, and Periodontal Disease: A Systematic Review", The Open Dentistry Journal, 2024.
Pan X, Wang Z, Wu X, Wen SW, Liu A. Salivary cortisol in post-traumatic stress disorder: a systematic review and meta-analysis. BMC Psychiatry. 2018 Oct 5;18(1):324. doi: 10.1186/s12888-018-1910-9. PMID: 30290789; PMCID: PMC6173866.
Basson R, O'Loughlin JI, Weinberg J, Young AH, Bodnar T, Brotto LA. Dehydroepiandrosterone and cortisol as markers of HPA axis dysregulation in women with low sexual desire. Psychoneuroendocrinology. 2019 Jun;104:259-268. doi: 10.1016/j.psyneuen.2019.03.001. Epub 2019 Mar 8. PMID: 30909007; PMCID: PMC7343293.
Hannibal KE, Bishop MD. Chronic stress, cortisol dysfunction, and pain: a psychoneuroendocrine rationale for stress management in pain rehabilitation. Phys Ther. 2014 Dec;94(12):1816-25. doi: 10.2522/ptj.20130597. Epub 2014 Jul 17. PMID: 25035267; PMCID: PMC4263906.
Lopez-Jornet P, Zavattaro E, Mozaffari HR, Ramezani M, Sadeghi M. Evaluation of the Salivary Level of Cortisol in Patients with Oral Lichen Planus: A Meta-Analysis. Medicina (Kaunas). 2019 May 27;55(5):213. doi: 10.3390/medicina55050213. PMID: 31137861; PMCID: PMC6571959.
Vandana S, Kavitha B, Sivapathasundharam B. Salivary cortisol and dehydroepiandrosterone as oral biomarkers to determine stress in patients with recurrent aphthous stomatitis. J Oral Maxillofac Pathol. 2019 May-Aug;23(2):213-217. doi: 10.4103/jomfp.JOMFP_282_18. PMID: 31516226; PMCID: PMC6714283.
Lennartsson AK, Sjörs A, Jonsdottir IH. Indication of attenuated DHEA-s response during acute psychosocial stress in patients with clinical burnout. J Psychosom Res. 2015 Aug;79(2):107-11. doi: 10.1016/j.jpsychores.2015.05.011. Epub 2015 May 21. PMID: 26071787.
Tananska VT. Salivary α-Amylase And Chromogranin A In Anxiety-Related Research. Folia Med (Plovdiv). 2014 Oct-Dec;56(4):233-6. doi: 10.1515/folmed-2015-0001. PMID: 26444351.
Mizuhashi F, Koide K, Toya S, Takahashi M, Mizuhashi R, Shimomura H. Levels of the antimicrobial proteins lactoferrin and chromogranin in the saliva of individuals with oral dryness. J Prosthet Dent. 2015 Jan;113(1):35-8. doi: 10.1016/j.prosdent.2013.12.028. Epub 2014 Oct 7. PMID: 25300178.
Saruta J, Tsukinoki K, Sasaguri K, Ishii H, Yasuda M, Osamura YR, Watanabe Y, Sato S. Expression and localization of chromogranin A gene and protein in human submandibular gland. Cells Tissues Organs. 2005;180(4):237-44. doi: 10.1159/000088939. PMID: 16330879.
Ball J, Darby I. Mental health and periodontal and peri-implant diseases. Periodontol 2000. 2022 Oct;90(1):106-124. doi: 10.1111/prd.12452. Epub 2022 Aug 1. PMID: 35913583; PMCID: PMC9804456.
Sahu GK, Upadhyay S, Panna SM. Salivary alpha amylase activity in human beings of different age groups subjected to psychological stress. Indian J Clin Biochem. 2014 Oct;29(4):485-90. doi: 10.1007/s12291-013-0388-y. Epub 2013 Oct 6. PMID: 25298630; PMCID: PMC4175699.
Jafari A, Pouramir M, Shirzad A, Motallebnejad M, Bijani A, Moudi S, Abolghasem-Zade F, Dastan Z. Evaluation of salivary alpha amylase as a biomarker for dental anxiety. Iranian Journal of Psychiatry and Behavioral Sciences. 2018 Mar 31;12(1).
Refulio Z, Rocafuerte M, de la Rosa M, Mendoza G, Chambrone L. Association among stress, salivary cortisol levels, and chronic periodontitis. J Periodontal Implant Sci. 2013 Apr;43(2):96-100. doi: 10.5051/jpis.2013.43.2.96. Epub 2013 Apr 30. PMID: 23678393; PMCID: PMC3651943.
Seizer L, Schubert C. On the Role of Psychoneuroimmunology in Oral Medicine. Int Dent J. 2022 Dec;72(6):765-772. doi: 10.1016/j.identj.2022.07.002. Epub 2022 Sep 30. PMID: 36184323; PMCID: PMC9676547.
Obulareddy VT, Chava VK, Nagarakanti S. Association of Stress, Salivary Cortisol, and Chronic Periodontitis: A Clinico-biochemical Study. Contemp Clin Dent. 2018 Sep;9(Suppl 2):S299-S304. doi: 10.4103/ccd.ccd_289_18. PMID: 30294161; PMCID: PMC6169263.
Rahate PS, Kolte RA, Kolte AP, Lathiya VN, Gupta M, Chari S. Evaluation of stress, serum, and salivary ghrelin and cortisol levels in smokers and non-smokers with Stage III periodontitis: A cross-sectional study. J Periodontol. 2022 Aug;93(8):1131-1140. doi: 10.1002/JPER.21-0373. Epub 2022 Jan 25. PMID: 34859428.
Zhang H, Chen B, Pan C, Zhang A. To evaluate the serum cortisol, salivary cortisol, and serum interleukin-1 B level in patients of chronic periodontitis with smoking and stress and without smoking and stress. Medicine (Baltimore). 2021 Aug 6;100(31):e26757. doi: 10.1097/MD.0000000000026757. PMID: 34397819; PMCID: PMC8341332.
Ponzio E, Dolcini J, Sparabombe S, Firmani G, D'Errico MM, Barbadoro P. Psychological Stress and its relationship to Periodontal flora and salivary Nitrite/Nitrate. Int Dent J. 2024 Aug;74(4):746-753. doi: 10.1016/j.identj.2024.02.003. Epub 2024 Mar 27. PMID: 38538383; PMCID: PMC11287180.
Mudrika S, Muthukumar S, Suresh R. Relationship between salivary levels of cortisol and dehydroepiandrosterone levels in saliva and chronic periodontitis. Journal of the International Clinical Dental Research Organization. 2014 Jul 1;6(2):92-7.