
Sorption-based Spectrophotometric Assay for Lead(II) with Immobilized Azo Ligand
Chemistry受け取った 03 Jan 2025 受け入れられた 22 Jan 2025 オンラインで公開された 23 Jan 2025
Focusing on Biology, Medicine and Engineering ISSN: 2995-8067 | Quick Google Scholar
Next Full Text
Erosion Corrosion of Commercially Pure Titanium and Ti-6Al-4V Alloy in Sodium Chloride Solutions with and Without Suspended Solids
Previous Full Text
Preventing Chronic Pain: Solutions to a Public Health Crisis


受け取った 03 Jan 2025 受け入れられた 22 Jan 2025 オンラインで公開された 23 Jan 2025
This study introduces a novel sorption-spectrophotometric method for the determination of lead(II) ions in water, addressing the critical need for sensitive and selective environmental monitoring. The method utilizes Amido Black (AB), a diazo dye, immobilized on a hexamethylenediamine-modified polyacrylonitrile fibrous support (PPD-1). The resulting immobilized reagent system (Pb(II)-AB/PPD-1) exhibits a significant bathochromic shift upon complexation with Pb(II), enabling spectrophotometric determination at 490 nm.
Optimal conditions for immobilization and complexation were established, including a pH range of 6-7 and an AB concentration of 8.2·10⁻⁵ M on the support. The method demonstrates a linear response to Pb(II) concentrations between 5 - 50 µg/ml (R² = 0.9938), with a low limit of detection (0.141) and quantification (0.47). Interference from common metal ions was minimal or mitigated with citric acid. Application to natural water samples showed excellent accuracy and precision (RSD ≤ 2.9).
The immobilized system offers a simplified procedure by eliminating elution steps, enhancing throughput, and achieving a ten-fold improvement in the limit of detection compared to solution-based methods. This rapid, sensitive, and selective method presents a promising alternative for lead(II) determination, offering comparable or superior performance with added benefits of simplicity and enhanced sensitivity.
Anthropogenic activities are a major contributor to environmental pollution, with the contamination of natural water sources being of particular concern. In Central Asia, characterized by limited water resources, the availability of potable water is a critical issue. Fresh water reserves in Uzbekistan's rivers and lakes are severely constrained, and groundwater and glacial sources, often considered a solution, are also susceptible to the accumulation of toxic pollutants. Thus, access to clean drinking water constitutes a strategic challenge for the 21st century. The World Health Organization has designated Heavy Toxic Metals (HTMs) – including cadmium, lead, mercury, arsenic, chromium, copper, and zinc – as priority pollutants requiring rigorous monitoring. Consequently, the detection and quantification of HTM ions in water are of paramount importance. Soil contamination by HTMs is also a significant concern due to their widespread anthropogenic discharge [-]. The relevance of this study lies in the established pathway of ecotoxicant entry into the human body: soil-plant-human-environment []. Effective environmental pollution monitoring is essential to mitigate anthropogenic impacts, thus highlighting the need for improved and comprehensive monitoring methods []. Sorption-spectrophotometric methods employing solid-phase immobilized organic reagents have emerged as promising alternatives to conventional solution-based spectrophotometry, exhibiting enhanced sensitivity and
selectivity [,]. Lead, a ubiquitous environmental contaminant from natural sources, such as galena, anglesite, and cerussite, is widely used in various industrial applications including batteries, cables, solder, and radiation shielding [].
These large-scale industries, often associated with significant waste generation and emissions, contribute to both atmospheric and waterborne lead pollution. Given the low permissible concentration limit of lead in drinking water (30 µg/L), continuous monitoring of lead contamination in various environmental matrices and water is imperative [,,].
Various optical techniques are employed for the analytical determination of lead in environmental matrices, including Atomic Absorption Spectrometry (AAS) and spectrophotometric methods utilizing diverse classes of organic reagents. Diazo reagents are among the most extensively studied in this context [-]. However, sorption-spectrophotometric methods for lead quantification remain relatively limited, with a scarcity of highly effective organic reagents specific for lead []. Given the inherent advantages of sorption-spectrophotometry, particularly its enhanced sensitivity for trace analysis of various toxicants, the development of robust and sensitive sorption-spectrophotometric methods for lead is a critical area of research. Recent advancements in improving the analytical performance of luminescent reagents have focused on the immobilization of organic ligands onto solid supports. This approach combines analyte preconcentration with in situ determination directly on the solid matrix [-].
The objective of this study was to establish a rapid and sensitive sorption-spectrophotometric method for the determination of lead(II) ions. This was achieved through the use of sodium 4-amino-5-hydroxy-3-((E)-(4-nitrophenyl)diazenyl)-6-((E)-phenyldiazenyl)naphthalene-2,7-disulfonate (AC), a diazoazo compound, immobilized on a fibrous support. The inherent selectivity of this reagent has enabled its application in lead analysis, even in the presence of numerous other metal ions. This article demonstrates the superior performance of sorption-spectrophotometric lead(II) determination using the immobilized organic reagent compared to conventional solution-based spectrophotometry.
Reagents and materials: Analytical reagents used were standard solutions. Amido Black 10B (systematic name: sodium 4-amino-5-hydroxy-3-((E)-(4-nitrophenyl)diazenyl)-6-((E)-phenyldiazenyl)naphthalene-2,7-disulfonate) was selected as the immobilized analytical reagent, obtained from "Ximreaktivinvest OOO," Tashkent, Uzbekistan (CAS number: 1064-48-8). The molecular structure of Amido Black is presented in Figure 1. A 1 × 10⁻⁵ M standard solution of Amido Black 10B was prepared in distilled water. Working solutions of the organic reagent, Amido Black, were produced by diluting stock solutions with bidistilled water. This standard solution was employed in immobilization procedures.
Lead standard solution: Pb(NO₃)₂ was also sourced from "Ximreaktivinvest OOO," Tashkent, Uzbekistan. A total of 1.598 g of Pb(NO₃)₂ was dissolved in distilled water and the volume was adjusted to 1 liter in a volumetric flask with distilled water, yielding a standard solution containing 1 mg Pb²⁺ per ml. This solution was utilized in the determination of Pb²⁺ as described in [].
Buffer solutions: pH values ranging from 1 to 10 were prepared using chemically pure-grade salts and acids, following previously described methodologies []. Throughout the study, freshly distilled and purified solvents, bidistilled water, and deionized water were employed, all verified to be non-luminescent.
Infrared (IR) spectra were recorded using both a UR-10 spectrophotometer (Carl Zeiss, Jena) and an Analitrsystem 360 FT-IR spectrometer (Nikolet Instrument Corporation, USA). Samples were analyzed in KBr and LiF pellets, as well as in CHCl3 solution, within the 500-4000 cm-1 range. pH measurements were conducted using a METTLER TOLEDO pH meter, calibrated with standard buffers in the same solvent used for analysis. Aqueous pH values for the buffer mixtures were referenced []. A PP-2-15 peristaltic pump was used for solution handling. Reagents of "chemically pure" and analytical grades were used. 0.1 M solutions of metal salts were prepared by dissolving the appropriate nitrate or chloride salts and further diluting them to obtain the desired concentrations. The organic reagent, Amido Black, was prepared by dissolving a specific amount of the reagent in a 100 mL volumetric flask.
Various fibrous materials, based on polyacrylonitrile, with different functional groups, were evaluated as solid supports. The sorbent was used in the form of disks, 20 mm in diameter and 30 mg - 40 mg in wet mass. These disks were pretreated with 0.1 N hydrochloric acid, washed with distilled water, and stored in Petri dishes.
Spectral analysis: Diffuse reflectance spectra of the solid surfaces were acquired using an X-Rite recording spectrophotocolorimeter. Reflectance (R) and reflectance function F(R) were also analyzed as functions of different variables using a dual-beam UV-Vis SPECORD 50 spectrophotometer.
The analytical signal was quantified as the change in diffuse reflectance (ΔR) measured at 610 nm. This value represents the difference in reflectance between the solid supports following analyte sorption and subsequent reaction with the immobilized reagent, and that of a blank solid support. The pH of the medium was monitored using an I-130 ionometer.
Experiments were conducted in both static and dynamic modes. In the static mode, 10.0 mL of the reagent solution was added to a 50.0 mL flask. A support disk was then immersed and stirred for 5-8 minutes. After this time the solution was decanted while retaining the solid support, which was then washed with distilled water and subsequently immersed in the analytical solution. In the dynamic mode, the analytical solution was passed through the immobilized support disk at a flow rate of 10 mL/min.
The retention of Amido Black on the solid support (R%) was quantified using the following equation: R% = 100 * (A / A0), where A represents the absorbance of the immobilized reagent after it was retained on the solid support, and A0 is the absorbance of the reagent before the immobilization process.
The diazo dye Amido Black (AB) demonstrates moderate sensitivity in its interaction with lead(II) ions. The complex molecular architecture of AB, illustrated in Figure 1, suggests a multifaceted reaction mechanism with metal ions that remains to be fully elucidated. Current hypotheses propose that this interaction involves a combination of ionic bonding and physical adsorption processes.
The accurate determination of lead is significantly affected by the presence of several interfering metal ions, including iron, aluminum, bismuth, and other metals. These interferences are typically mitigated through the introduction of masking agents and by optimizing the acidity of the reaction medium.
Several fibrous materials, functionalized with various anion-exchange groups, were evaluated as potential solid-phase supports for Amido Black immobilization. Among the materials tested, the fibrous support modified with hexamethylenediamine (designated PPD-1) exhibited superior analytical performance, demonstrating the effective complexation of Amido Black. Optimal immobilization of Amido Black within the PPD-1 polymer matrix was observed within a pH range of 3.0 to 6.0. Due to its effective retention of Amido Black, the PPD-1 support, forming the PPD-1:AB complex (hereafter referred to as IMAB), was selected for subsequent experimentation.
The impact of both the Amido Black concentration and the immobilization time was examined over a range of 1·10-6
to 1·10-3 M Amido Black solution and 3 to 30 minutes, respectively (Table 1).
Table 1 details the optimal conditions for the determination of Pb(II) utilizing the immobilized Amido Black organic reagent. The maximum reflectance spectrum (λMeR) of the immobilized Amido Black organic reagent with Pb(II) is observed at 490 nm, while the reflectance spectrum (λR) of the immobilized Amido Black reagent occurs at 610 nm. The optimal pH range for complex formation is determined to be between 6 and 7. The immobilization of Amido Black on the fiber is achieved within 7 minutes. The concentration of Amido Black immobilized on 0.2 g PPD-1 fiber is quantified as 8.2·10-5 M. Figure 2 depicts the reflectance spectra of the complex formed by Amido Black immobilized on PPD-1 fiber and the immobilized Amido Black with Pb(II).
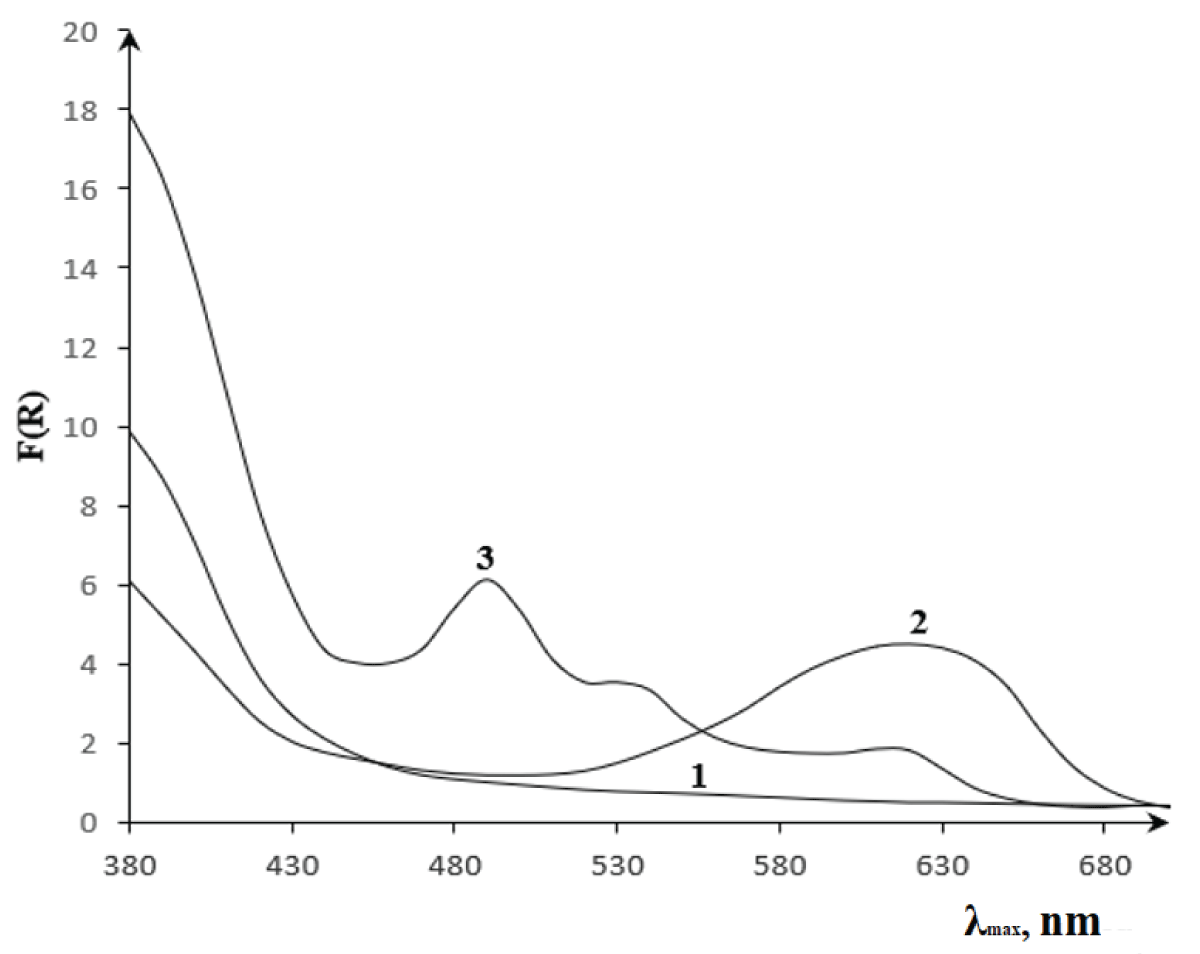 Figure 2: Reflectance spectra of the complex formed by fiber (1), immobilized reagent (2) with lead (II) ion (3) in a universal buffer mixture.
Figure 2: Reflectance spectra of the complex formed by fiber (1), immobilized reagent (2) with lead (II) ion (3) in a universal buffer mixture.Upon immobilization on the PPD-1 support, the complexation of Amido Black (AB) with Pb(II) resulted in a substantial bathochromic shift of 120 nm (Δλ = 120 nm), indicating a significant alteration in the electronic structure compared to the reaction in solution. The maximum reflectance wavelength (λMeR) of the resulting Pb(II)-AB complex on the PPD-1 was observed at 490 nm. Comparatively, other reagents reported for Pb (II) determination, including 4-(Pyridyl-2-azo)-resorcinol (520 nm) [], 4-(Thiazolyl-2-azo)-resorcinol (540 nm) [,], Dithizone (520 nm) [,], Arsenazo III (605 nm) [], Xylenol Orange (580 nm) [], Bromopyrogallol Red (630 nm) [], Glycine Thymol Blue (574 nm) [], Methylthymol Blue (600 nm) [], Sulfonazo III (650 nm) [], KI + Methyl Green (642 nm) [], and Sulfarsazene (510 nm) [], exhibit λmax values generally higher than that of the Pb(II)-AB/PPD-1 complex. However, the observed λMeR for the immobilized system falls within a similar spectral region. This suggests that while the immobilized AB system exhibits a unique spectral response upon interaction with Pb(II), its performance, in terms of maximum wavelength, is comparable to established reagents.
The dependence of the analytical signal on Pb(II) concentration for the complex formed between Pb(II) and Amido Black immobilized on the PPD-1 matrix (Pb(II)-AB/PPD-1) is depicted in Figure 3. Experimental conditions were maintained as follows: V = 100 mL, m = 0.2 g AB/PPD-1, pH = 6.5 ± 0.5, T = 20 ± 2 °C, and t = 20 min. The investigation spanned a Pb(II) concentration range of 5-50 µg/ml A strong linear correlation was observed within this range, as indicated by a regression coefficient (R²) of 0.9938. This linearity validates the accurate quantification of Pb(II) ions using the Amido Black reagent immobilized on the PPD-1 support and suggests the suitability of this immobilized reagent system for quantitative analysis within the specified 5-50 µg/ml Pb(II) concentration range.
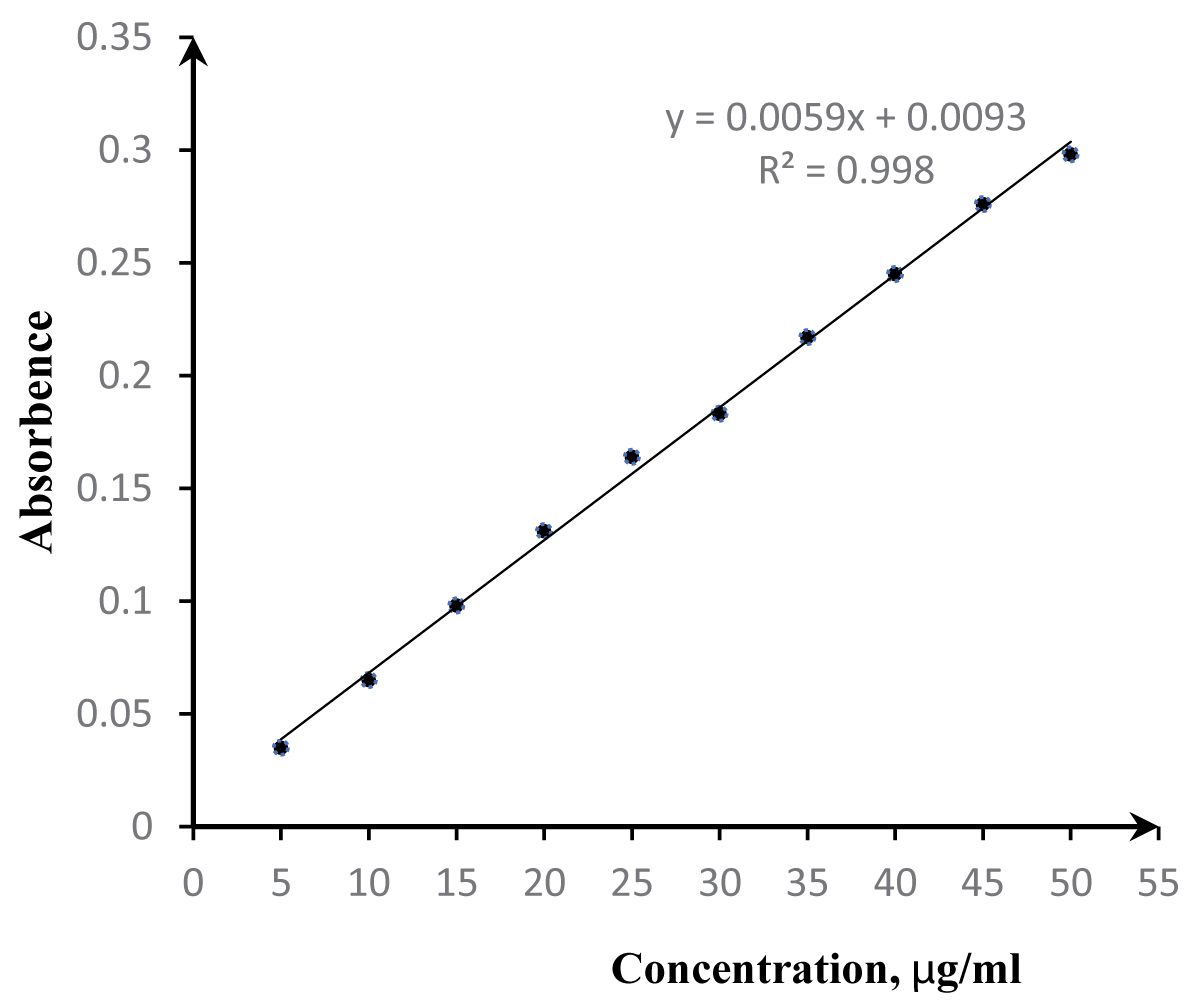 Figure 3: Dependence of the optical density of the Pb(II) complex with the AB/PPD-1 matrix on the Pb2+ concentration (V = 100 ml, m = 0.2 g AB/PPD-1 matrix, pH = 6.5 ± 0.5, T = 20 ± 2 °C), t = 20 min).
Figure 3: Dependence of the optical density of the Pb(II) complex with the AB/PPD-1 matrix on the Pb2+ concentration (V = 100 ml, m = 0.2 g AB/PPD-1 matrix, pH = 6.5 ± 0.5, T = 20 ± 2 °C), t = 20 min).It was determined that the presence of Cd(II), Mn(II), Ni(II), Co(II), Zn(II), and Mg(II) at concentrations up to 100 mg/L does not interfere with the quantification of lead(II). The interference caused by Fe(III), Bi(III), and Al(III) at concentrations of 50 mg/L was successfully mitigated through the addition of 0.1 M citric acid solution to the sample. A method for the determination of lead(II) in natural water samples was developed. The accuracy of this method was verified using a spike-recovery assay, as presented in Table 2. The analysis time was determined to be 10-15 minutes. The pH range for the reaction is similar to that reported for 4-(Thiazolyl-2-azo)-resorcinol (6.5-7) [,] and Bromopyrogallol Red (4-7) [].
The analytical method demonstrated high precision, with a Relative Standard Deviation (RSD) consistently ≤ 2.9. The limit of detection (LOD) and limit of quantification (LOQ) were determined to be 0.141 and 0.47, respectively, while method reproducibility was 1.67. Notably, the use of Amido Black immobilized on fibrous supports enabled selective lead analysis without the need for an elution step. This streamlined procedure enhanced the analytical throughput and resulted in a ten-fold improvement in the LOD compared to the presented method. Furthermore, this approach offers advantages over existing methods utilizing chromogenic reagents such as 4-(Pyridyl-2-azo)-resorcinol [], Dithizone [,], and Sulfarsazene [], which require higher pH conditions for optimal performance.
Conclusion
This study demonstrates the successful immobilization of Amido Black (AB) on a hexamethylenediamine-modified polyacrylonitrile fibrous support (PPD-1) for the sensitive and selective determination of lead(II) ions in aqueous solutions. The resulting immobilized reagent system, denoted as Pb(II)-AB/PPD-1, exhibited a significant bathochromic shift upon complexation with Pb(II), allowing for its spectrophotometric determination at a λMeR of 490 nm. Optimal immobilization and complexation were achieved within a pH range of 6-7, with an immobilization time of 7 minutes and an AB concentration of 8.2·10-5 M on the PPD-1 support.
The method exhibited a strong linear response to Pb(II) concentrations between 5-50 µg/ml, with a regression coefficient (R²) of 0.9938, demonstrating its suitability for quantitative analysis. The presence of potentially interfering ions such as Cd(II), Mn(II), Ni(II), Co(II), Zn(II), and Mg(II) did not affect the determination of Pb(II) at concentrations up to 100 mg/L. The interference of Fe(III), Bi(III), and Al(III) was effectively eliminated using citric acid as a masking agent.
The developed method was successfully applied to the determination of Pb(II) in natural water samples, showing excellent accuracy and precision, with a Relative Standard Deviation (RSD) consistently ≤ 2.9. The method exhibited a low limit of detection (LOD) of 0.141 and a limit of quantification (LOQ) of 0.47, demonstrating its high sensitivity. The reproducibility was determined to be 1.67.
Importantly, the immobilized system offers a simplified analytical procedure by eliminating the need for an elution step, thereby enhancing throughput and achieving a ten-fold improvement in the LOD compared to the standard solution-based method. Furthermore, the optimized pH range for complexation (6-7) offers an advantage over other established chromogenic reagents, which often require higher pH conditions.
In conclusion, the developed method utilizing Amido Black immobilized on PPD-1 presents a rapid, sensitive, selective, and reliable approach for the determination of lead (II) in environmental samples. This novel immobilized reagent system offers a promising alternative to existing methods, providing comparable or superior performance with the added benefits of simplicity, speed, and enhanced sensitivity. Further research could explore the application of this system to other matrices and the immobilization of other analytical reagents for diverse analytical applications.
Moor JV, Ramamurthy S. Heavy metals in natural waters. Moscow: Mir. 1987;297.
Remezov TB. Medicinal chemistry of elements. Leningrad. 1978;95.
Polyakov EV, Egorov YV. Modern methods for determining the physicochemical state of trace elements in natural waters. Uspekhi Chem. 2003;72(11):1103-1114.
Gil'denskiol'd RS, Novikov IuV, Khamidulin RS, Aniskina RI, Vinokur IL. Tiazhelye metally v okruzhaiushcheĭ srede i ikh vliianie na organizm (obzor) [Heavy metals in the environment and their effects on the body (review of the literature)]. Gig Sanit. 1992 May-Jun;(5-6):6-9. Russian. PMID: 1398184.
Gerlach SA. Pollution of the seas: Diagnosis and therapy. Leningrad: Hydrometeoizdat. 1985.
Mudry IV. Heavy metals in the soil-plant-human system (review). Hygiene and Sanitation. 1997;(1):16-19.
Zolotov YA, Kuzmin NM, Neiman EY, Popov AA, Revalsky IA. The concept of chemical-analytical control of environmental objects. Russ Chem J. 1993;77(64):12-16.
Smanova ZA, Usmanova HU. Immobilization of oxyazo compounds to improve the metrological parameters of sorption-spectroscopic determination of some metals. Uzb Chem J. 2018;(3):89-95.
Akl MA. An improved colorimetric determination of lead(II) in the presence of nonionic surfactant. Anal Sci. 2006 Sep;22(9):1227-31. doi: 10.2116/analsci.22.1227. PMID: 16966814.
Smanova ZA, Usmanova H. Determination of beryllium by solid-phase spectroscopy. Bull NUU. Nat Sci. 2017;(3/2):469-471.
Smanova ZA, Usmanova HU. Sorption-luminescent determination of heavy metals using immobilized morin. Rep Acad Sci Repub Uzbekistan. 2016;(6):59-61.
Korostelev PP. Preparation of solutions for chemical analytical work. 1964.
Lurie YY. Handbook of analytical chemistry. Moscow: Chemistry. 1989;267-275.
Dagnall RM, West TS, Young P. Determination of lead with 4-(2-pyridylazo)-resorcinol—I: Spectrophotometry and solvent extraction. Talanta. 1965;12(6):583-588.
Temerev SV, Loginova OB. Extraction-voltammetric method for determining zinc, cadmium, lead, and copper in natural waters.
Malakhova NM. Abstract of a Candidate of Chemical Sciences Dissertation. Odessa.
Geiger LW, Sandel EB. Anal. Chim Acta. 1953;1:197-200.
Mathre OB, Sandell EB. II Talanta. 1964;9(2):295-314.
Vesene TE, Bagulene ZP. Chemistry, technology, physics, geography. 1979;67-70.
Pochinok TB, Anisimovich PV, Temerdashev ZA, Reshetnyak EA. Sorption-spectroscopic determination of Pb (II) with bromopyrogallol red immobilized in hardened gelatin gel. Analyt Control. 2013;(4):477-484.
Temerdashev ZA, Pochinok TB, Tarasova PV, Gosteva MA. Study of immobilization of bromopyrogallol red in a gelatin matrix and assessment of the possibility of creating an optically transparent sensor for metal detection on its basis. Analyt Control. 2012;(1):39-45.
Yakovenko AV, Bevz OV, Ruban EA. Development of methods for the identification and quantitative determination of glycine in an experimental dosage form. Med Acad South Kazakhstan Bull. 2020;5(4):91.
Kostenko EE. Solid-phase spectrophotometric determination of Pb (II) using methylthymol blue. Mod Sci Intensive Technol. 2007;(12):1-6.
Kostenko EE, Christiansen MG, Butenko EN. Photometric determination of trace amounts of lead in drinking water using sulfonazo III.
Balogh YS. Formation, properties, extraction, and analytical application of ionic associates involving cyanine and other basic dyes. (Doctoral dissertation). Odessa: A. V. Bogatsky Institute of Physical Chemistry, Ukraine Academy of Sciences. 1995.
Kostenko EE. Solid-phase spectrophotometric determination of lead with chromazurol S. J Anal Chem. 2010;65:366-370.
Allanazarovich AM, Rajabboyevna YM, Rajabovich TY, Asanaliyevna SZ, Akbarovich AM. Sorption-based Spectrophotometric Assay for Lead(II) with Immobilized Azo Ligand. IgMin Res. January 23, 2025; 3(1): 052-056. IgMin ID: igmin283; DOI:10.61927/igmin283; Available at: igmin.link/p283
次のリンクを共有した人は、このコンテンツを読むことができます:
1Senior Scientific Researcher at the Khorezm Ma’mun Academy, PhD in Chemistry Sciences, Uzbekistan
2Junior Scientific Researcher at the Khorezm Ma’mun Academy, Uzbekistan
3Urgench State University, Faculty of Natural and Agricultural Sciences, Urgench, Uzbekistan
4Professor of the Chemistry Faculty of the National University of Uzbekistan, Tashkent, Uzbekistan
Address Correspondence:
Ashirov Mansur Allanazarovich, Senior Scientific Researcher at the Khorezm Ma’mun Academy, PhD in Chemistry Sciences, Uzbekistan, Email: [email protected]
How to cite this article:
Allanazarovich AM, Rajabboyevna YM, Rajabovich TY, Asanaliyevna SZ, Akbarovich AM. Sorption-based Spectrophotometric Assay for Lead(II) with Immobilized Azo Ligand. IgMin Res. January 23, 2025; 3(1): 052-056. IgMin ID: igmin283; DOI:10.61927/igmin283; Available at: igmin.link/p283
Copyright: © 2025 Allanazarovich AM, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
 Figure 1: Structural formula of the Amido Black reagent....
Figure 1: Structural formula of the Amido Black reagent....
 Figure 2: Reflectance spectra of the complex formed by fiber...
Figure 2: Reflectance spectra of the complex formed by fiber...
 Figure 3: Dependence of the optical density of the Pb(II) co...
Figure 3: Dependence of the optical density of the Pb(II) co...
 Table 1: Spectrophotometric characteristics of amido black ...
Table 1: Spectrophotometric characteristics of amido black ...
 Table 2: Lead (II) determination results in water samples (...
Table 2: Lead (II) determination results in water samples (...
Moor JV, Ramamurthy S. Heavy metals in natural waters. Moscow: Mir. 1987;297.
Remezov TB. Medicinal chemistry of elements. Leningrad. 1978;95.
Polyakov EV, Egorov YV. Modern methods for determining the physicochemical state of trace elements in natural waters. Uspekhi Chem. 2003;72(11):1103-1114.
Gil'denskiol'd RS, Novikov IuV, Khamidulin RS, Aniskina RI, Vinokur IL. Tiazhelye metally v okruzhaiushcheĭ srede i ikh vliianie na organizm (obzor) [Heavy metals in the environment and their effects on the body (review of the literature)]. Gig Sanit. 1992 May-Jun;(5-6):6-9. Russian. PMID: 1398184.
Gerlach SA. Pollution of the seas: Diagnosis and therapy. Leningrad: Hydrometeoizdat. 1985.
Mudry IV. Heavy metals in the soil-plant-human system (review). Hygiene and Sanitation. 1997;(1):16-19.
Zolotov YA, Kuzmin NM, Neiman EY, Popov AA, Revalsky IA. The concept of chemical-analytical control of environmental objects. Russ Chem J. 1993;77(64):12-16.
Smanova ZA, Usmanova HU. Immobilization of oxyazo compounds to improve the metrological parameters of sorption-spectroscopic determination of some metals. Uzb Chem J. 2018;(3):89-95.
Akl MA. An improved colorimetric determination of lead(II) in the presence of nonionic surfactant. Anal Sci. 2006 Sep;22(9):1227-31. doi: 10.2116/analsci.22.1227. PMID: 16966814.
Smanova ZA, Usmanova H. Determination of beryllium by solid-phase spectroscopy. Bull NUU. Nat Sci. 2017;(3/2):469-471.
Smanova ZA, Usmanova HU. Sorption-luminescent determination of heavy metals using immobilized morin. Rep Acad Sci Repub Uzbekistan. 2016;(6):59-61.
Korostelev PP. Preparation of solutions for chemical analytical work. 1964.
Lurie YY. Handbook of analytical chemistry. Moscow: Chemistry. 1989;267-275.
Dagnall RM, West TS, Young P. Determination of lead with 4-(2-pyridylazo)-resorcinol—I: Spectrophotometry and solvent extraction. Talanta. 1965;12(6):583-588.
Temerev SV, Loginova OB. Extraction-voltammetric method for determining zinc, cadmium, lead, and copper in natural waters.
Malakhova NM. Abstract of a Candidate of Chemical Sciences Dissertation. Odessa.
Geiger LW, Sandel EB. Anal. Chim Acta. 1953;1:197-200.
Mathre OB, Sandell EB. II Talanta. 1964;9(2):295-314.
Vesene TE, Bagulene ZP. Chemistry, technology, physics, geography. 1979;67-70.
Pochinok TB, Anisimovich PV, Temerdashev ZA, Reshetnyak EA. Sorption-spectroscopic determination of Pb (II) with bromopyrogallol red immobilized in hardened gelatin gel. Analyt Control. 2013;(4):477-484.
Temerdashev ZA, Pochinok TB, Tarasova PV, Gosteva MA. Study of immobilization of bromopyrogallol red in a gelatin matrix and assessment of the possibility of creating an optically transparent sensor for metal detection on its basis. Analyt Control. 2012;(1):39-45.
Yakovenko AV, Bevz OV, Ruban EA. Development of methods for the identification and quantitative determination of glycine in an experimental dosage form. Med Acad South Kazakhstan Bull. 2020;5(4):91.
Kostenko EE. Solid-phase spectrophotometric determination of Pb (II) using methylthymol blue. Mod Sci Intensive Technol. 2007;(12):1-6.
Kostenko EE, Christiansen MG, Butenko EN. Photometric determination of trace amounts of lead in drinking water using sulfonazo III.
Balogh YS. Formation, properties, extraction, and analytical application of ionic associates involving cyanine and other basic dyes. (Doctoral dissertation). Odessa: A. V. Bogatsky Institute of Physical Chemistry, Ukraine Academy of Sciences. 1995.
Kostenko EE. Solid-phase spectrophotometric determination of lead with chromazurol S. J Anal Chem. 2010;65:366-370.