High Resolution X-ray Diffraction Studies of the Natural Minerals of Gas Hydrates and Occurrence of Mixed Phases
Materials Science Structural EngineeringEnergy Systems受け取った 02 Feb 2024 受け入れられた 01 Nov 2024 オンラインで公開された 04 Nov 2024
Focusing on Biology, Medicine and Engineering ISSN: 2995-8067 | Quick Google Scholar
Next Full Text
Technical & Economic Feasibility Study of Proposed Pump Storage Power Plants at Kuda Oya, Mul Oya, Gurugal Oya, and Dambagasthalawa

受け取った 02 Feb 2024 受け入れられた 01 Nov 2024 オンラインで公開された 04 Nov 2024
The types and concentrations of gas hydrates collected from four different geological sites were analyzed using high-resolution angular dispersive X-ray powder diffraction, with synchrotron radiation as the source. Measurements were taken across a temperature range of 80 to 300 K and a pressure range of 0.1 to 80 MPa. All four gas hydrate samples showed the presence of three mixed crystal structures: structures I, II, and H. Additionally, the ice Ih structure was inherently present in all the natural gas hydrate minerals. The variation in the types and concentrations of gas hydrates can be attributed to the diversity of natural gases at different geographic locations.
Gas hydrates form when natural gases migrate from beneath the seafloor along natural faults and precipitate or crystallize upon contact with cold seawater at pressures of several tens of MPa [-]. Although gas hydrates can be synthesized in the laboratory, the experiments suggest the formation of a single phase due to the controlled growth parameters and the reactants in nature. For example, laboratory experiments can be controlled to produce a clathrate hydrate of natural gas in a purely single structure type [-]. However, achieving this in nature is rarely possible. This is because the crystalline states obtained on the seafloor depend on four major geophysical, organo-chemical, and microbiological field variables: (1) the type and concentration of natural gases present and their migration rates, (2) the temperature and pressure of seafloor water, (3) the concentration of living and non-living non-water bodies, and (4) the prevailing hydrodynamics and other physical variables of the seafloor water environment.
Gas hydrates, or clathrate hydrates of natural gases, are solid-like minerals formed at low temperatures and high pressures by van der Waals forces between gas and water molecules. The host water molecules form molecular cages that trap the guest gas molecules through mutual electrostatic interactions. These hydrates contain natural gases, such as hydrocarbons, which are encapsulated within the water molecule cages.
Although clathrate hydrates of natural gases contain colorless hydrocarbons, not all of them appear milky white like snow. For instance, hydrates from the Gulf of Mexico are often richly colored in shades of yellow, orange, or red, while samples from the Cascadia Margin are almost entirely milky white. The origin of the coloration is not fully understood, but it is believed that the presence of oil, bacteria, and minerals all play a role. In the ocean, gas hydrates, which are predominantly composed of methane, are common constituents of the shallow marine geosphere and occur both as deep sedimentary structures and as outcrops on the ocean floor.
Natural gas hydrates are found globally in marine sediments, permafrost regions, and continental ice sheets. Their formation depends on the presence of sufficiently high concentrations of natural gases such as methane and more complex hydrocarbons, along with elevated pressures and reduced temperatures. Methane is primarily produced through the fermentative decomposition of organic matter or the bacterial reduction of CO2 in sediments. The thermo-catalytic conversion of organic matter in the deeper subsurface can also be an equally important source of methane and more complex hydrocarbons. In this case, methane may migrate from deeper sources into the hydrate stability zone []. Large hydrate deposits are found along continental margins, where their formation is favored by rapid sedimentation and high sedimentary organic matter content. On land, stable methane hydrates in permafrost regions likely result from the natural migration of gases from deeper hydrocarbon reservoirs. Methane hydrates are also found in polar ice sheets, forming at depth due to air inclusions [].
Hydrocarbons captured in natural gas hydrates predominantly consist of methane [-]. Based on their sizes and their ability to interact with water, the condensed matter state of hydrophobic (hydrocarbon) gas molecules with water can be classified into five types [-]:
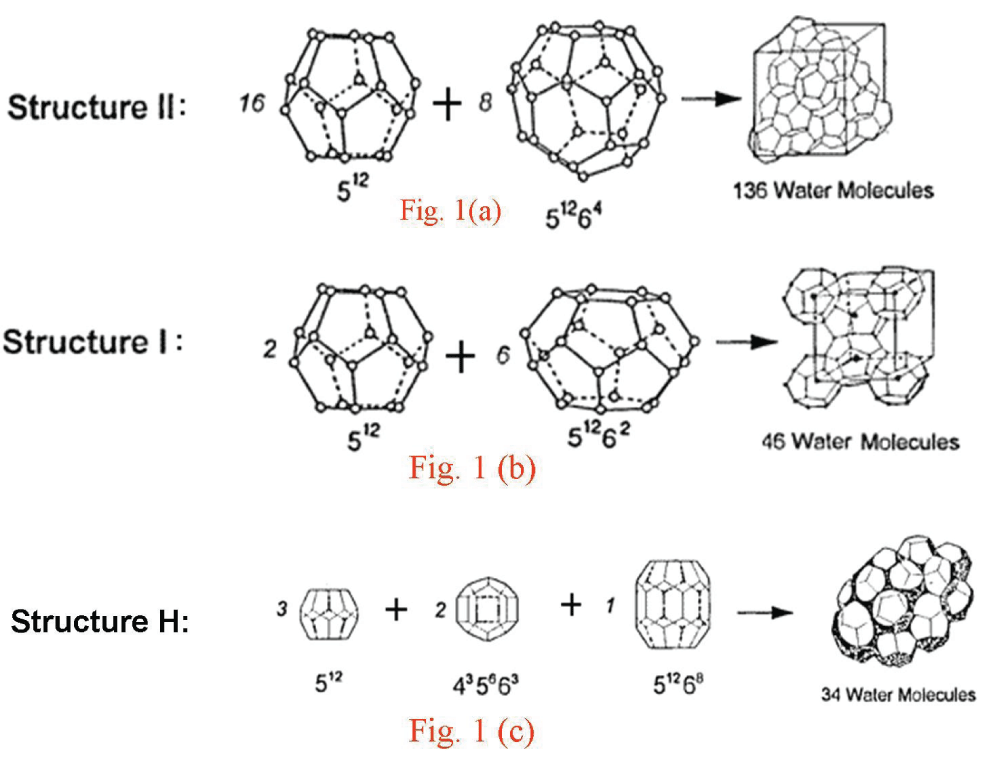 Figure 1: Unit cells of hydrate structures II (a), I (b), and H (c). The building blocks are in the form of cages formed by water molecules. Structures I and II have two types of cages; structure H has three types of cages.
Figure 1: Unit cells of hydrate structures II (a), I (b), and H (c). The building blocks are in the form of cages formed by water molecules. Structures I and II have two types of cages; structure H has three types of cages.The research presented in this paper focuses on the study of clathrate hydrates of natural gases collected from various geographic locations in the Pacific, Atlantic, and Arctic Oceans. This study was undertaken for three main reasons: (1) structural information is crucial for designing and developing technology to extract this extensive frozen energy resource from the seafloor in an economically viable manner, (2) comprehensive structural data on these natural minerals are currently lacking to our knowledge, and (3) laboratory experiments have not fully replicated the complex natural processes responsible for hydrate formation. Our research specifically emphasizes structural investigations of these natural clathrate hydrate minerals.
Using Department of Navy submarine vessels, clathrate hydrates of natural gases were collected from five distinct geographical locations: (1) the Texas-Louisiana Shelf in the Gulf of Mexico, (2) the Nankai Trough off the Eastern Coast of Japan, (3) the Blake Ridge in the Northwestern Atlantic Ocean, (4) the Cascadia Margin in the Northeastern Pacific Ocean, and (5) the Hakkon-Mosby Mud Volcano in the Norwegian-Greenland Sea. During submarine dives, naturally occurring gas hydrates were retrieved from approximately 600 meters depth. These hydrates were transferred into plastic bags and stored in an LN2 dewar to maintain their natural state.
A flat, sturdy metallic platform, kept near LN2 temperature, supported a pestle-mortar combination used to finely powderize the hydrates from the LN2 dewar. The resulting powder was filtered through a fine wire-mesh (<200 µm) to isolate the desired samples. An aluminum piston-cylinder assembly, with dimensions of 12 mm inner diameter and 25 mm length, capable of withstanding pneumatic pressures up to ~100 MPa, was loaded with the sample and promptly transferred to a closed cycle refrigerator (CCR) attached to the BESSRC CAT ID 11-D beamline at the Advanced Photon Source (APS), Argonne National Laboratory. The 11-ID-D beamline features a modified BESSRC double crystal monochromator covering an energy range of 4.0 to 40 keV, utilizing a cryo-cooled Si(220) crystal with a 20 mm fixed offset. The incident beam was focused by a Pt-coated toroidal mirror with a 2.8 mrad incident angle []. The CCR maintained sample temperatures between 80 and 300 K, with pressure and temperature stabilities observed at 0.01 MPa and 1 K, respectively.
Temperature variations across the sample were monitored using two thermocouples placed at the ends of the pressure cell, ensuring a temperature difference of less than 3 K before data acquisition. The incident beam energy was optimized at 25 keV (λ = 0.49592 Å) to achieve the best resolution, largest interplanar spacing, and highest signal-to-noise ratio.
Initial experiments involved blank cell measurements to establish peak positions using aluminum as the reference. Subsequently, X-ray powder standards of Al, Al2O3, and ice were employed for diffractometer calibration to ensure precision and accuracy. Lattice parameter resolution was approximately 10-4 Å with accuracy better than 10-3 Å.
High-resolution X-ray diffraction data were acquired using a scintillation counter detector positioned approximately 300 mm from the sample. Data collection utilized a 2θ-step of 0.01° with each point measured for 3 seconds within the range 1.5° ≤ 2θ ≤ 30°. Following sample pressurization with high-purity nitrogen gas (4 to 80 MPa), accurate 2θ values and peak intensities were determined using XFIT, a peak profile-fitting program. The majority of diffraction peaks were fitted with pseudo-Voigt distributions, while some required Gaussian and Lorentzian fits. XRDA, a least squares fitting program, was used to analyze the diffraction data sets, identifying peaks corresponding to ice Ih, and clathrate hydrate structures sI, sII, and sH.
Structure refinements incorporated lattice parameters from XRDA output, symmetry positions based on NMR findings, and atomic positions (oxygen, half-hydrogen, carbon, and hydrogen) sourced from single crystal X-ray and neutron diffraction literature, employing covalent radii throughout the refinement process. Iterative refinement steps were undertaken to optimize agreement between experimental and observed intensity data.
First, we describe the hydrocarbon composition and stable carbon isotope data on the hydrate minerals. Since, the formation, stability and dissociation energy of hydrates are functions of biological, chemical, and physical conditions, we performed these two measurements. The hydrocarbon composition data are presented in Table 1 and it is noted that the hydrate minerals consist of a broad range of gases. The stable carbon isotope analyses for hydrocarbons (CH4 to C4H10) and CO2 indicate that in the samples from the Gulf of Mexico and Haakon Mosby Mud Volcano, the value of d13C in CH4 is in the range of -60 to -50, implying that these minerals have biogenic origin. In the following, representative structural results on natural minerals of gas hydrates obtained from three geographical locations are presented.
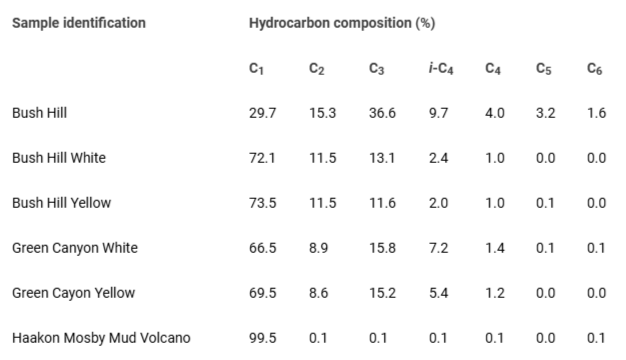 Table 1: Hydrocarbon content in hydrate samples from the Taxas-Louisiana Shelf in the Gulf of Mexico and the Haakon-Mosby Mud Volcano in the Norwegian Greenland Sea. Bush Hill and Green Canyon are located in the Gulf of Mexico. Yellow hydrates have Petroleum present between the clathrate structures.
Table 1: Hydrocarbon content in hydrate samples from the Taxas-Louisiana Shelf in the Gulf of Mexico and the Haakon-Mosby Mud Volcano in the Norwegian Greenland Sea. Bush Hill and Green Canyon are located in the Gulf of Mexico. Yellow hydrates have Petroleum present between the clathrate structures.The natural minerals collected from the Northern Pacific Ocean Play were found to exhibit a notable concentration of methane, attributed to a biogenic origin. A representative diffractogram at a temperature of 150 K is depicted in Figure 2.
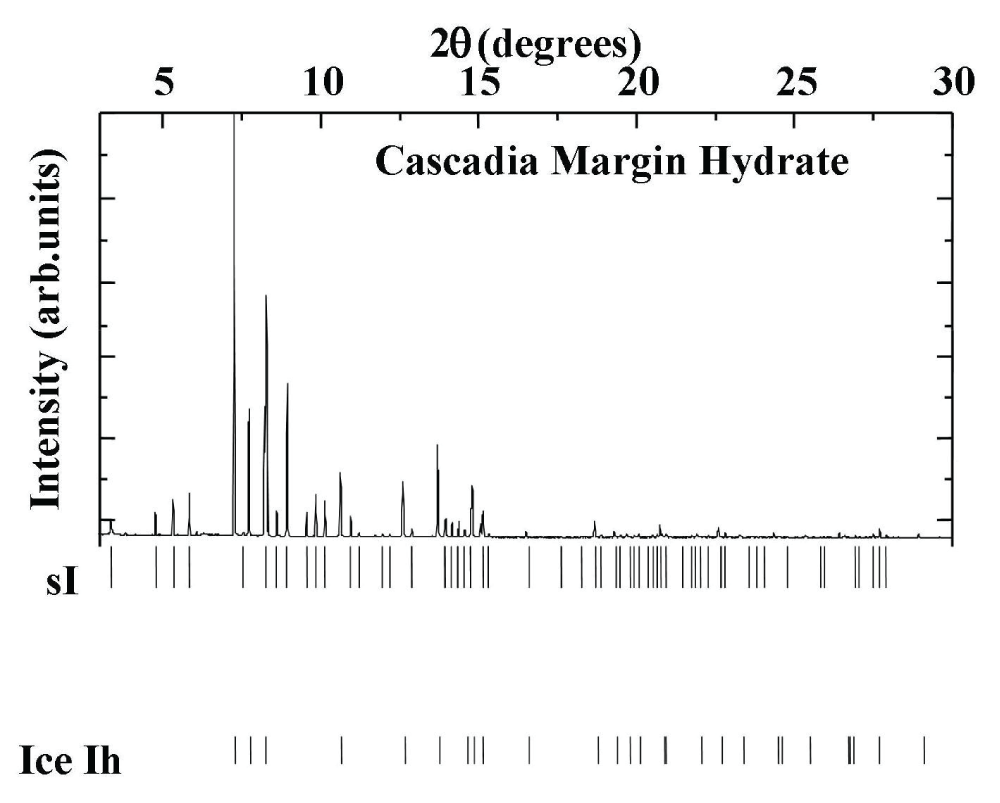 Figure 2: Powder diffraction pattern for the hydrate sample of Cascadia Margin, showing major constituents are ice Ih and hydrate sI.
Figure 2: Powder diffraction pattern for the hydrate sample of Cascadia Margin, showing major constituents are ice Ih and hydrate sI.Table 2 lists the experimental d-spacings. The lattice parameters for ice Ih (Space Group P63/mmc) are: a = 4.5115 ± 0.0068 Å and c = 7.3566 ± 0.0001 Å. For the clathrate hydrate in sI, the lattice parameter is a = 11.9132 ± 0.0235 Å. The predominant structure of the clathrate hydrates of natural gas samples obtained from the Cascadia Margin is cubic, with the space group Pm3n. It is noteworthy that natural clathrate hydrates from the Pacific seafloor, collected during the research cruise SONNE 110, were found to predominantly contain sI structure, with the sample comprising 97.4% CH4, 2.6% H2S, and traces of CO2, C4H6, and C3H8 [].
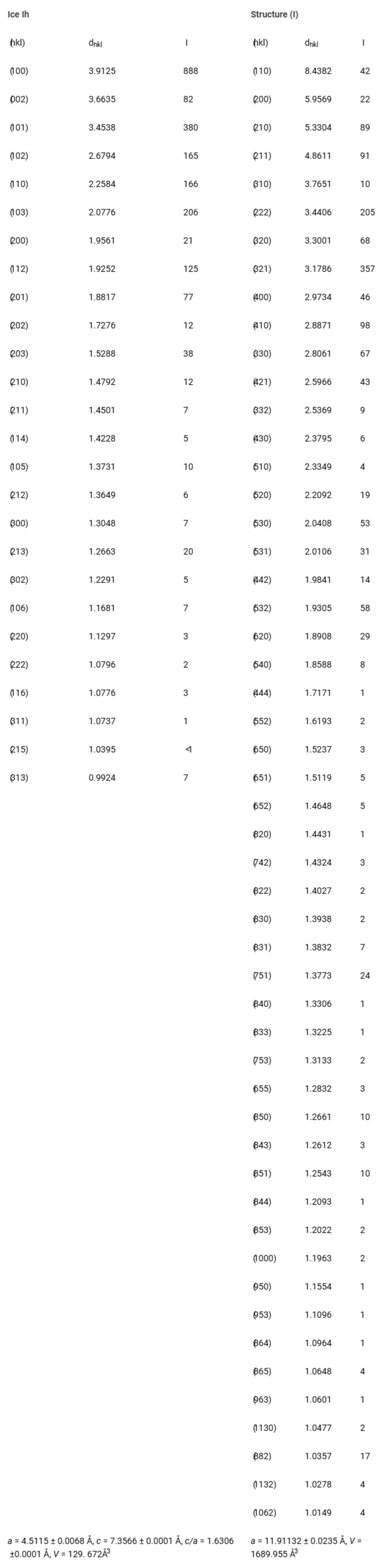 Table 2: Powder diffraction data analysis of the Cascadia Margin Sample at P = 5.6 MPa, T = 85 K. In the 2θ range 1.6 to 300, 78 discernible peaks are observed, of which 26 peaks are due to ice Ih and 52 peaks due to sI.
Table 2: Powder diffraction data analysis of the Cascadia Margin Sample at P = 5.6 MPa, T = 85 K. In the 2θ range 1.6 to 300, 78 discernible peaks are observed, of which 26 peaks are due to ice Ih and 52 peaks due to sI.The Jolliet Field in Green Canyon 184 exemplifies a direct association between oil accumulation and thermogenic gas hydrate. Oil and gas are trapped in Pleistocene-Pliocene reservoir sands at depths of approximately 2-3 kilometers. The Bush Hill gas hydrate site, located on GC185 at the surface trace of a hydrocarbon-charged antithetic fault (27° 47.5' N, 91° 30.5' W), is near the Jolliet Field at a depth of about 540 meters. This natural mineral was collected off the southwestern coast of the United States. Analysis of this Gulf of Mexico mineral revealed it contains 72 to 74% methane, with the remainder being higher hydrocarbons (Table 1). A typical diffractogram (T = 150 K) is shown in Figure 3, and Table 3 lists the experimental d-spacings. The lattice parameters for ice Ih are: a = 4.5047 ± 0.00037 Å, c = 7.2412 ± 0.0001 Å, while the lattice parameter for sII is: a = 17.0888 ± 0.0004 Å. Although the Bush Hill samples primarily consist of sII and ice Ih, several peaks may originate from sI (a = 11.7346 ± 0.0300 Å) and sH (a = 11.4496 ± 0.3324 Å, c = 9.7064 ± 0.0330 Å), as detailed in Table 3.
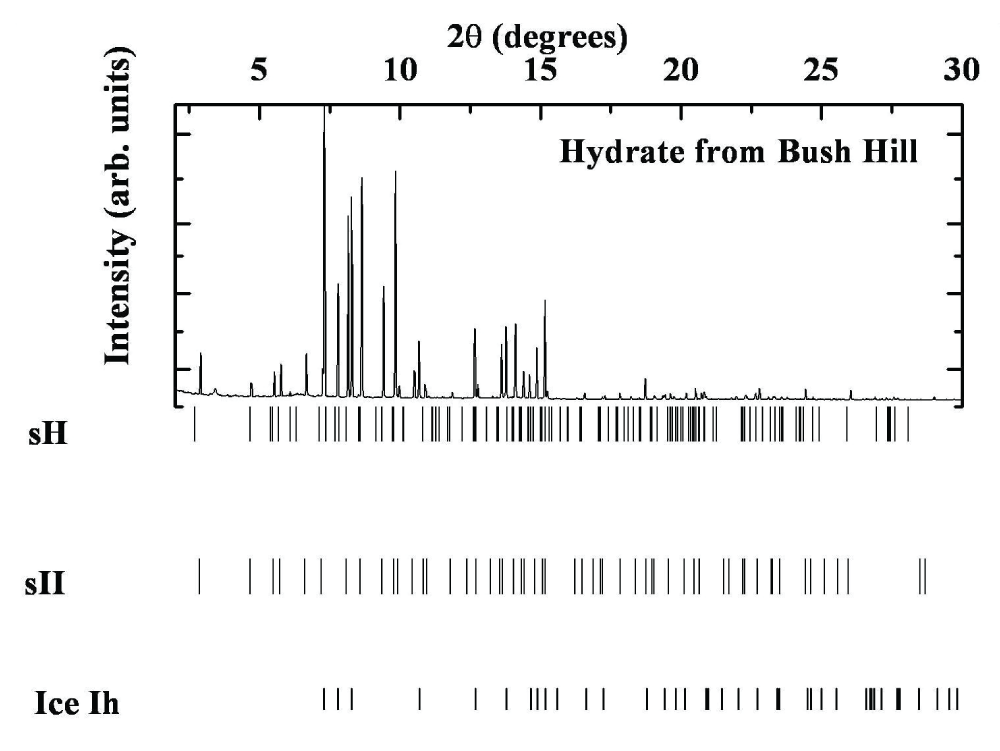 Figure 3: X-ray Powder diffraction pattern for the hydrate sample from Bush Hill, Gulf of Mexico. The major structures of the sample are ice Ih and hydrate sII.
Figure 3: X-ray Powder diffraction pattern for the hydrate sample from Bush Hill, Gulf of Mexico. The major structures of the sample are ice Ih and hydrate sII.The Green Canyon, Gulf of Mexico minerals have been analyzed to contain 66 to 70% methane, with the remainder consisting of higher hydrocarbons, particularly ethylene and cyclohexane. A typical diffractogram (T = 150 K) is shown in Figure 4, and Table 4 lists the experimental d-spacings. The lattice parameters for ice Ih are: a = 4.5170 ± 0.00032 Å, c = 7.3665 ± 0.0001 Å, while the lattice parameter for structure II (sII) is: a = 17.2170 ± 0.0060 Å. Although the Green Canyon samples primarily consist of sII and ice Ih, several peaks suggest the presence of structure I (sI) with lattice parameters a = 11.9292 ± 0.0380 Å and structure H (sH) with lattice parameters a = 11.7284 ± 0.0451 Å and c = 9.6717 ± 0.0001 Å, as detailed in Table 4.
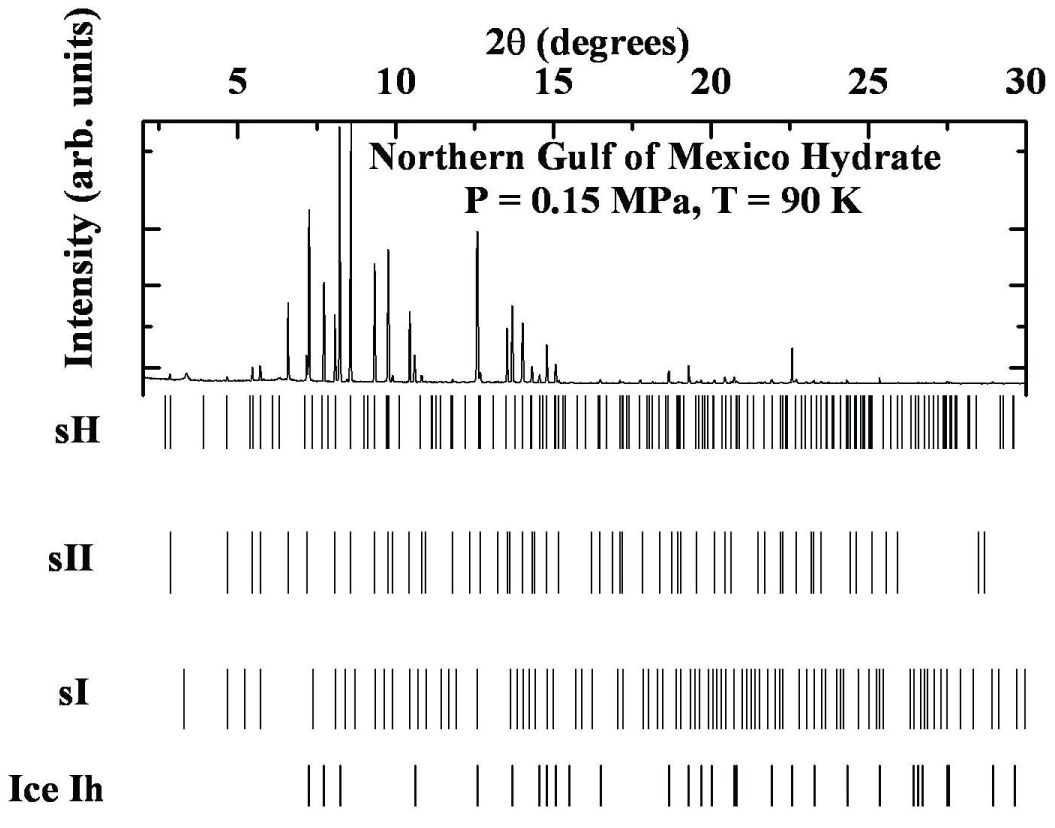 Figure 4: Powder diffraction pattern for the hydrate sample from Green Canyon, Northern Gulf of Mexico. The major structures of the sample are ice Ih and hydrate sII. A major conclusion gleaned from the powder pattern is that the quantity of hydrate samples is much more than ice.
Figure 4: Powder diffraction pattern for the hydrate sample from Green Canyon, Northern Gulf of Mexico. The major structures of the sample are ice Ih and hydrate sII. A major conclusion gleaned from the powder pattern is that the quantity of hydrate samples is much more than ice.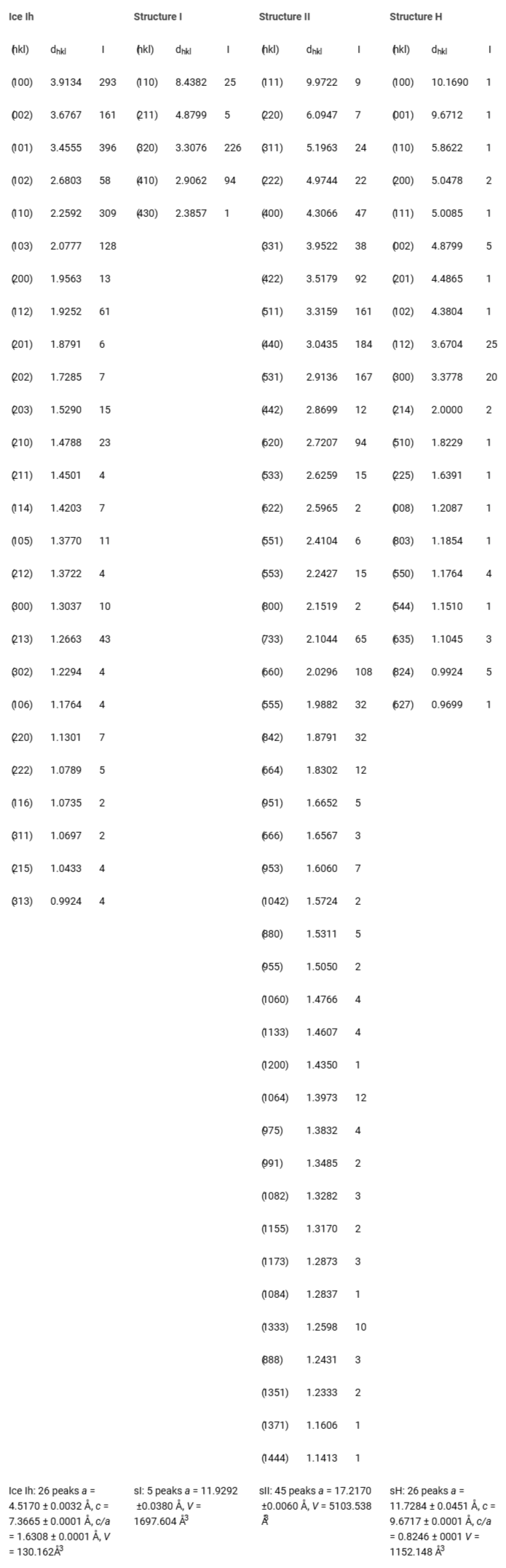 Table 4: Powder diffraction data analysis of the hydrate sample from the Northern Gulf of Mexico at P = 0.15 MPa, T = 90 K. In the 2θ range 1.60 to 300, 102 peaks are observed of which 26 peaks are due to ice Ih, 45 peaks due to sII, and remaining 26 and 5 peaks can correspondingly be assigned to sH and sI.
Table 4: Powder diffraction data analysis of the hydrate sample from the Northern Gulf of Mexico at P = 0.15 MPa, T = 90 K. In the 2θ range 1.60 to 300, 102 peaks are observed of which 26 peaks are due to ice Ih, 45 peaks due to sII, and remaining 26 and 5 peaks can correspondingly be assigned to sH and sI.Spurred by this work, several theoretical and experimental efforts have ensued. Notable ones are summarized as follows: Using grand canonical Monte Carlo simulations, Papadimitriou et al have shown that above 380 MPa, 3.0 wt% hydrogen storage could be achieved []. The study of Bourry et al on natural hydrates from the African margin indicates a preponderance of biogenic methane with a minor composition of CO2 and H2S and at elevated temperatures, the thermal expansion approached that of ice []. Raman spectroscopic investigation on hydrates extracted from the West African margin in the South Atlantic Ocean (ZaiAngo and Neris II) and from Hakon Mosby Mud Volcano (Norwegian Sea) exhibit a major concentration of CH4 [].
Present studies carried out on the hydrate samples recovered from Bush Hill and Green Canyon show the existence of a minor sH structure. Future studies such as high energy high-resolution nuclear techniques are needed to clearly identify the natural gases present in natural hydrates.
In the present study, structural analyses of natural gas hydrate minerals were conducted using high-resolution angular dispersive X-ray diffraction at the BESSRC CAT ID 11-D beam-line of the Advanced Photon Source. It was determined that the gas hydrate samples from the Cascadia Margin consist of structure I (sI) and ice Ih, while those from Bush Hill and Green Canyon contain structure II (sII) and ice Ih, along with traces of structure H (sH). Ice Ih is found to coexist in all the natural mineral samples. The variation in the concentration of sI, sII, and sH in these samples indicates significant differences in the types and concentrations of natural gases present at these geographically distinct locations. The stronger ice peaks in the Cascadia Margin samples suggest a lower concentration of hydrates compared to the Bush Hill and Green Canyon samples, which contain more than 60% hydrates.
We are grateful to Drs. Ken Grabowski, David Knies, and B.B. Rath for encouragement and helpful discussions. Also, we acknowledge the assistance of Dr. Jennifer A. Linton, BESSRC CAT, Materials Science Division, Argonne National Laboratory in setting up the experiments.
Coffin RB, Lamontagne R, Rose-Pehrsson S, Grabowski KS, Knies DL, Qadri SB, Yesinowski JP, Pohlman JW, Yousuf M, Linton JA. Ocean Floor Methane Gas Hydrate Exploration. NRL Review. 2002;112.
Yousuf M, Qadri SB, Knies DL, Grabowski KS, Coffin RB, Pohlman JW. Novel results on structural investigations of natural minerals of clathrate hydrates. Appl Phys A. 2003;78:925-939.
Haq BU. Methane in the Deep Blue Sea. Science. 1999;285:543-544.
Brooks JM, Kennicutt II MC, Fay RR, McDonald TJ, Sassen R. Thermogenic Gas Hydrates in the Gulf of Mexico. Science. 1984;225:409-411.
Kennicutt II MC, Brooks JM, Denoux GJ. Leakage of deep, reservoired petroleum to the near surface on the Gulf of Mexico continental slope. Marine Chem. 1988;24:39-59.
MacDonald IR, Guinasso NL Jr, Sassen R, Brooks JM, Lee L, Scott KT. Gas hydrate that breaches the sea floor on the continental slope of the Gulf of Mexico. Geology. 1994;22:699-702.
McMullan RK, Jeffery GA. Polyhedral Clathrate Hydrates. IX. Structure of Ethylene Oxide Hydrate. J Chem Phys. 1965;42:2725-2732.
Mak TCW, McMullan RK. Polyhedral Clathrate Hydrates. X. Structure of the Double Hydrate of Tetrahydrofuran and Hydrogen Sulfide. J Chem Phys. 1965;42:2732-2737.
Udachin KA, Ripmeester JA. A complex clathrate hydrate structure showing bimodal guest hydration. Nature. 1999;397:420-423.
Ripmeester JA, Ratcliffe CI. The diverse nature of dodecahedral cages in clathrate hydrates as revealed by 129Xe and 13C NMR spectroscopy: CO2 as a small-cage guest. Energy Fuels. 1998;12:197-200.
Sloan ED. Gas Hydrates: Review of Physical/Chemical Properties. Energy Fuels. 1998;12:191.
Holbrook WS, Hoskins H, Wood WT, Stephen RA, Lizarralde D. Methane Hydrate and Free Gas on the Blake Ridge from Vertical Seismic Profiling. Science. 1996;273:1840-1843.
Shoji H, Langway CC. Air hydrate inclusions in fresh ice core. Nature. 1982;298:548-550.
Makogon YF, Makogon TY, Holditch SA. Gas Hydrate: Challenges for the Future. In: Holder GD, Bishnoi PR, editors. Annals of the New York Academy of Sciences. 2000;912:777.
Rogner HH. An Assessment of World Hydrocarbon Resources. Annu Rev Energy Environ. 1997;22:217-262.
Sassen R, MacDonald IR, Requejo AG, Guinasso NL Jr, Kennicutt II MC, Sweet ST, Brooks JM. Organic geochemistry of sediments from chemosynthetic communities, Gulf of Mexico slope. Geo-Marine Lett. 1994;14:110-119.
Pauling L. The Nature of the Chemical Bond and The Structure of Molecules and Crystals. Ithaca, NY: Cornell University Press; 1960.
Jeffrey GA. Inclusion Compounds. Vol. 1: Structural Aspects of Inclusion Compounds Formed by Inorganic and Organometallic Host Lattices. Atwood JL, Davies JED, MacNichol DD, editors. Academic Press; 1984;135.
Sloan ED Jr. Clathrate Hydrates of Natural Gases. New York: Marcel Dekker, Inc.; 1998.
Rahman A, Stillinger FH. Hydrogen-bond patterns in liquid water. J Am Chem Soc. 1973;95:7943.
Khan A. Theoretical studies of tetrakaidecahedral structures of (H2O)24, (H2O)25 and (H2O)26 clusters. Chem Phys Lett. 1996;253:299-304.
Beno MA, Kurtz C, Munkholm A, Rüt U, Engbretson M, Jennings G, Linton J, Knapp GS, Montano PA. Elliptical multipole wiggler beamlines at the advanced photon source. Nucl Instrum Methods Phys Res A. 2001;467-468:694.
Gutt C. Europhys Lett. 1999;48:269.
Papadimitriou NI, Tsimpanogiannis IN, Papaioannou A Th, Stubos AK. Evaluation of the Hydrogen-Storage Capacity of Pure H2 and Binary H2-THF Hydrates with Monte Carlo Simulations. J Phys Chem C. 2008;112:10294.
Bourry C, Chazallon B, Charlou JL, Donval JP, Ruffine L, Henry P, Geli L, Çagatay MN, İnan S, Moreau M. Free gas and gas hydrates from the Sea of Marmara, Turkey: Chemical and structural characterization. Geophys Res Lett. 2009;264:197-206.
Chazallon B, Focsa C, Charlou JL, Bourry C, Donval JP. A comparative Raman spectroscopic study of natural gas hydrates collected at different geological sites. Chem Geol. 2007;244:175-185
Yousuf M, Qadri S. High Resolution X-ray Diffraction Studies of the Natural Minerals of Gas Hydrates and Occurrence of Mixed Phases. IgMin Res.. November 04, 2024; 2(11): 889-896. IgMin ID: igmin265; DOI:10.61927/igmin265; Available at: igmin.link/p265
次のリンクを共有した人は、このコンテンツを読むことができます:
1The George Washington University, Washington, DC 20052, USA
2Emeritus, U. S. Naval Research Laboratory, Washington, DC 20375, USA
Address Correspondence:
SB Qadri, Emeritus, U. S. Naval Research Laboratory, Washington, DC 20375, USA, Email: [email protected]
How to cite this article:
Yousuf M, Qadri S. High Resolution X-ray Diffraction Studies of the Natural Minerals of Gas Hydrates and Occurrence of Mixed Phases. IgMin Res.. November 04, 2024; 2(11): 889-896. IgMin ID: igmin265; DOI:10.61927/igmin265; Available at: igmin.link/p265
Copyright: © 2024 Yousuf M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
 Figure 1: Unit cells of hydrate structures II (a), I (b), an...
Figure 1: Unit cells of hydrate structures II (a), I (b), an...
 Figure 2: Powder diffraction pattern for the hydrate sample ...
Figure 2: Powder diffraction pattern for the hydrate sample ...
 Figure 3: X-ray Powder diffraction pattern for the hydrate s...
Figure 3: X-ray Powder diffraction pattern for the hydrate s...
 Figure 4: Powder diffraction pattern for the hydrate sample ...
Figure 4: Powder diffraction pattern for the hydrate sample ...
 Table 1: Hydrocarbon content in hydrate samples from the Ta...
Table 1: Hydrocarbon content in hydrate samples from the Ta...
 Table 2: Powder diffraction data analysis of the Cascadia M...
Table 2: Powder diffraction data analysis of the Cascadia M...
 Table 3: Powder diffraction data analysis of the Bush Hill ...
Table 3: Powder diffraction data analysis of the Bush Hill ...
 Table 4: Powder diffraction data analysis of the hydrate sa...
Table 4: Powder diffraction data analysis of the hydrate sa...
Coffin RB, Lamontagne R, Rose-Pehrsson S, Grabowski KS, Knies DL, Qadri SB, Yesinowski JP, Pohlman JW, Yousuf M, Linton JA. Ocean Floor Methane Gas Hydrate Exploration. NRL Review. 2002;112.
Yousuf M, Qadri SB, Knies DL, Grabowski KS, Coffin RB, Pohlman JW. Novel results on structural investigations of natural minerals of clathrate hydrates. Appl Phys A. 2003;78:925-939.
Haq BU. Methane in the Deep Blue Sea. Science. 1999;285:543-544.
Brooks JM, Kennicutt II MC, Fay RR, McDonald TJ, Sassen R. Thermogenic Gas Hydrates in the Gulf of Mexico. Science. 1984;225:409-411.
Kennicutt II MC, Brooks JM, Denoux GJ. Leakage of deep, reservoired petroleum to the near surface on the Gulf of Mexico continental slope. Marine Chem. 1988;24:39-59.
MacDonald IR, Guinasso NL Jr, Sassen R, Brooks JM, Lee L, Scott KT. Gas hydrate that breaches the sea floor on the continental slope of the Gulf of Mexico. Geology. 1994;22:699-702.
McMullan RK, Jeffery GA. Polyhedral Clathrate Hydrates. IX. Structure of Ethylene Oxide Hydrate. J Chem Phys. 1965;42:2725-2732.
Mak TCW, McMullan RK. Polyhedral Clathrate Hydrates. X. Structure of the Double Hydrate of Tetrahydrofuran and Hydrogen Sulfide. J Chem Phys. 1965;42:2732-2737.
Udachin KA, Ripmeester JA. A complex clathrate hydrate structure showing bimodal guest hydration. Nature. 1999;397:420-423.
Ripmeester JA, Ratcliffe CI. The diverse nature of dodecahedral cages in clathrate hydrates as revealed by 129Xe and 13C NMR spectroscopy: CO2 as a small-cage guest. Energy Fuels. 1998;12:197-200.
Sloan ED. Gas Hydrates: Review of Physical/Chemical Properties. Energy Fuels. 1998;12:191.
Holbrook WS, Hoskins H, Wood WT, Stephen RA, Lizarralde D. Methane Hydrate and Free Gas on the Blake Ridge from Vertical Seismic Profiling. Science. 1996;273:1840-1843.
Shoji H, Langway CC. Air hydrate inclusions in fresh ice core. Nature. 1982;298:548-550.
Makogon YF, Makogon TY, Holditch SA. Gas Hydrate: Challenges for the Future. In: Holder GD, Bishnoi PR, editors. Annals of the New York Academy of Sciences. 2000;912:777.
Rogner HH. An Assessment of World Hydrocarbon Resources. Annu Rev Energy Environ. 1997;22:217-262.
Sassen R, MacDonald IR, Requejo AG, Guinasso NL Jr, Kennicutt II MC, Sweet ST, Brooks JM. Organic geochemistry of sediments from chemosynthetic communities, Gulf of Mexico slope. Geo-Marine Lett. 1994;14:110-119.
Pauling L. The Nature of the Chemical Bond and The Structure of Molecules and Crystals. Ithaca, NY: Cornell University Press; 1960.
Jeffrey GA. Inclusion Compounds. Vol. 1: Structural Aspects of Inclusion Compounds Formed by Inorganic and Organometallic Host Lattices. Atwood JL, Davies JED, MacNichol DD, editors. Academic Press; 1984;135.
Sloan ED Jr. Clathrate Hydrates of Natural Gases. New York: Marcel Dekker, Inc.; 1998.
Rahman A, Stillinger FH. Hydrogen-bond patterns in liquid water. J Am Chem Soc. 1973;95:7943.
Khan A. Theoretical studies of tetrakaidecahedral structures of (H2O)24, (H2O)25 and (H2O)26 clusters. Chem Phys Lett. 1996;253:299-304.
Beno MA, Kurtz C, Munkholm A, Rüt U, Engbretson M, Jennings G, Linton J, Knapp GS, Montano PA. Elliptical multipole wiggler beamlines at the advanced photon source. Nucl Instrum Methods Phys Res A. 2001;467-468:694.
Gutt C. Europhys Lett. 1999;48:269.
Papadimitriou NI, Tsimpanogiannis IN, Papaioannou A Th, Stubos AK. Evaluation of the Hydrogen-Storage Capacity of Pure H2 and Binary H2-THF Hydrates with Monte Carlo Simulations. J Phys Chem C. 2008;112:10294.
Bourry C, Chazallon B, Charlou JL, Donval JP, Ruffine L, Henry P, Geli L, Çagatay MN, İnan S, Moreau M. Free gas and gas hydrates from the Sea of Marmara, Turkey: Chemical and structural characterization. Geophys Res Lett. 2009;264:197-206.
Chazallon B, Focsa C, Charlou JL, Bourry C, Donval JP. A comparative Raman spectroscopic study of natural gas hydrates collected at different geological sites. Chem Geol. 2007;244:175-185