Quality Culture - Lessons Learned from the Low- and Medium Income World
Hematology RehabilitationPublic Health受け取った 09 Sep 2024 受け入れられた 26 Oct 2024 オンラインで公開された 28 Oct 2024
Focusing on Biology, Medicine and Engineering ISSN: 2995-8067 | Quick Google Scholar
Previous Full Text
Communication Training at Medical School: A Quantitative Analysis

受け取った 09 Sep 2024 受け入れられた 26 Oct 2024 オンラインで公開された 28 Oct 2024
With the development of safe blood supply and transfusion comes the introduction of quality as a culture, implemented through the introduction of a Quality System (QS) and Quality Management System (QMS). Often the idea is that– ‘when instructions are written (SOPs) a quality system is in place. Just follow the instructions, that is it.’ However, quality only partly depends on following instructions at the operational level. Generally not understood is the importance of designing and implementing a quality management system, based on 5 key elements 1) organization and structure; 2) standards (technical and quality); 3) documentation - traceability and evidence; 4) education - continued teaching and training; 5) assessment - continued monitoring and evaluation.
Development and implementation of an appropriate quality system and management were evaluated for 15 developing countries (2004-2020) in 4 WHO regions. Projects were based on a step-by-step introduction to the concept and principles of quality following a Quality Management Training (QMT) course – modular, interactive, with improvement score evaluation focused on comprehension and ownership of the teaching and training contents.
For an optimal understanding of the values of quality in Transfusion Medicine (TM), a culture has to be created - ownership development, commitment to and implementation of the principles of fitness for purpose, and the supplier-producer-customer continuum.
With the development of a safe blood supply and transfusion comes the introduction of quality as a culture, implemented through the implementation of a Quality System (QS) and Quality System Management (QSM) []. However, in many situations, the common idea is – ‘when operational instructions are written (SOPs) a quality system is in place. Just follow the instructions, that is it.’ However, quality only partly depends on following instructions at the operational level.
Generally not understood is the importance of designing and implementing a quality system, based on a Quality Management System (QMS) consisting of 5 key elements:
1) Organization and structure – mission and vision, policy and strategies;
2) Standards - technical and quality;
3) Documentation - traceability and evidence;
4) Education - continued teaching and training;
5) Assessment - continued monitoring and evaluation.
For an optimal understanding of the values of quality in transfusion medicine (TM), a 24/7 culture has to be created - ownership and stewardship development, commitment to and implementation of the principles of fitness for purpose, and the supplier-producer-customer continuum [-].
Development and implementation of an appropriate quality system and management were evaluated for 15 developing countries (2004-2020) in 4 WHO regions (Table 1). Projects were based on a step-by-step introduction of the concept and principles of quality following a Quality Management Training (QMT) course [] – modular, interactive, with improvement-score evaluation focused on comprehension and ownership of the teaching and training contents.
The WHO QMT consists of two parts (part 1 - basic principles of quality and part 2 - applying quality in the blood transfusion system) with 13 modules, 7 basic and 6 applied, and a completion Module (Table 2). Each module consists of lectures alternated by group activities to illustrate and implement the educated knowledge.
The QMT course outcomes were evaluated by comparing and analyzing the pre-course assessment outcomes with the post-course outcomes. That provides both the strong as well as the weak elements in understanding.
Since the introduction of WHO QMT courses in the 4 WHO regions, hundreds of blood establishment and healthcare facility professionals have been exposed.
However, often the selected trainees in these 15 countries were not properly prepared for the objectives and contents of such QMT courses, trainers and facilitators in the 4 WHO Regions had insufficient personal drive and experience, enthusiasm, and competence to conduct such high-level quality management course, and with regard to sustainability in-country follow up was not anticipated by existing leadership and therefore failed.
Nevertheless, a quality culture started to develop, and consistency of fitness for purpose and the supplier-producer-customer chain development as a healthcare-integrated managerial and operational system was noticed where –
Careful analysis of the outcomes of the QMT courses (post-course assessment) displayed important lessons to learn what the weak and what the strong elements were, in order to improve on the approaches for sustained development. When teaching focuses on outcomes (OBE) rather than on curriculum [,] the emphasis shifts from practical skills to a thoughtful understanding of quality culture, being more convinced of conducting active teamwork and implementing the supplier-producer-customer continuum and the fit-for-purpose principle.
Although education (teaching of and training} in principles of quality as a manageable system to improve blood safety and availability is important, it is not enough to create a quality culture needed for sustained and guaranteed quality operations in blood establishments and hospitals/healthcare facilities. Learning should be outcome-based rather than curriculum-oriented.
An important lesson learned is that the approach shall be holistic and supported by competent leadership – commitment and stewardship, ownership, and full intellectual understanding. The outcome then needs an active and continued in-country follow-up to allow proper and sustained implementation of knowledge to build on the creation of a 24/7 quality culture based on stewardship. In fact, the QMT course presents a learning tool for implementing SWOT (Strengths, Weaknesses, Opportunities, and Threads) lessons that will strengthen the fit-for-purpose principle and the awareness of the continuum of the supplier-producer-customer chain.
Holmberg JA, Rosin N. Need for quality management in transfusion medicine. In: Smit Sibinga CTh, editor. Quality management in transfusion medicine. New York: Nova Science Publishers; 2013;35-53.
Smit Sibinga CTh, Hasan F. Quality management or the need for a quality culture in transfusion medicine. Glob J Transfus Med. 2020;5(1):9-16.
Smit Sibinga CTh, Seidl C, Nunes E, AuBuchon JP. Quality and quality management systems applicable in transfusion medicine. In: Smit Sibinga CTh, editor. Quality management in transfusion medicine. New York: Nova Biomedical; 2013.
Abdella YE, Smit Sibinga CTh. WHO and current global efforts in transfusion medicine education. In: Eichbaum QG, editor. Global education, training, and staffing in transfusion medicine. Bethesda, MD: AABB Press; 2024 (in press).
World Health Organization. Quality management training for blood transfusion services: facilitator’s toolkit. Geneva: WHO; 2004. WHO/EHT/04.13.
Louw VJ. Determining the outcomes for clinicians completing a postgraduate diploma in transfusion medicine. Transfus Apher Sci. 2014 Dec;51(3):38-43. doi: 10.1016/j.transci.2014.10.009. Epub 2014 Oct 16. PMID: 25457005.
Louw VJ, Barrett CA, Rambiritch V. Outcomes-based clinical transfusion medicine education. In: Smit Sibinga CTh, editor. Clinical use of blood: an alternative approach. Switzerland: Springer Nature; 2024 (in press).
Sibinga CTS. Quality Culture - Lessons Learned from the Low- and Medium Income World. IgMin Res. October 28, 2024; 2(10): 870-872. IgMin ID: igmin262; DOI:10.61927/igmin262; Available at: igmin.link/p262
次のリンクを共有した人は、このコンテンツを読むことができます:
Address Correspondence:
Cees Th Smit Sibinga, MD, PhD, FRCP Edin, FRCPath, Professor, International Development of Transfusion Medicine, University of Groningen and IQM Consulting, The Netherlands, Email: [email protected]
How to cite this article:
Sibinga CTS. Quality Culture - Lessons Learned from the Low- and Medium Income World. IgMin Res. October 28, 2024; 2(10): 870-872. IgMin ID: igmin262; DOI:10.61927/igmin262; Available at: igmin.link/p262
Copyright: © 2024 Smit Sibinga CT. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
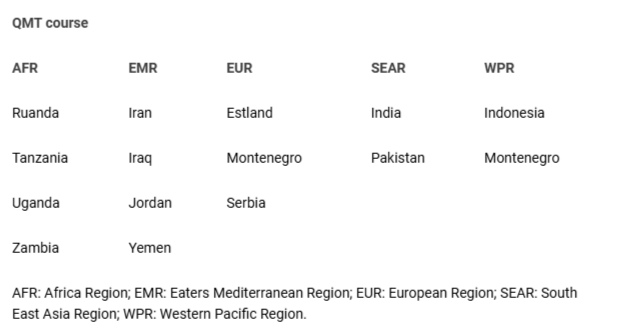 Table 1: QMT course: Low and Middle-Income Countries (LMIC)...
Table 1: QMT course: Low and Middle-Income Countries (LMIC)...
 Table 2: Modular Curriculum QMT course....
Table 2: Modular Curriculum QMT course....
Holmberg JA, Rosin N. Need for quality management in transfusion medicine. In: Smit Sibinga CTh, editor. Quality management in transfusion medicine. New York: Nova Science Publishers; 2013;35-53.
Smit Sibinga CTh, Hasan F. Quality management or the need for a quality culture in transfusion medicine. Glob J Transfus Med. 2020;5(1):9-16.
Smit Sibinga CTh, Seidl C, Nunes E, AuBuchon JP. Quality and quality management systems applicable in transfusion medicine. In: Smit Sibinga CTh, editor. Quality management in transfusion medicine. New York: Nova Biomedical; 2013.
Abdella YE, Smit Sibinga CTh. WHO and current global efforts in transfusion medicine education. In: Eichbaum QG, editor. Global education, training, and staffing in transfusion medicine. Bethesda, MD: AABB Press; 2024 (in press).
World Health Organization. Quality management training for blood transfusion services: facilitator’s toolkit. Geneva: WHO; 2004. WHO/EHT/04.13.
Louw VJ. Determining the outcomes for clinicians completing a postgraduate diploma in transfusion medicine. Transfus Apher Sci. 2014 Dec;51(3):38-43. doi: 10.1016/j.transci.2014.10.009. Epub 2014 Oct 16. PMID: 25457005.
Louw VJ, Barrett CA, Rambiritch V. Outcomes-based clinical transfusion medicine education. In: Smit Sibinga CTh, editor. Clinical use of blood: an alternative approach. Switzerland: Springer Nature; 2024 (in press).