
Use of Augmented Reality as a Radiation-free Alternative in Pain Management Spinal Surgeries
Pain and Relief受け取った 04 Jul 2024 受け入れられた 08 Aug 2024 オンラインで公開された 09 Aug 2024
ISSN: 2995-8067 | Quick Google Scholar
Next Full Text
Compatibility Analysis of Frequency Containment Reserve and Load Frequency Control Functions


受け取った 04 Jul 2024 受け入れられた 08 Aug 2024 オンラインで公開された 09 Aug 2024
Recent advancements in computer-assisted surgical navigation have enhanced imaging precision while reducing fluoroscopy reliance. However, integrating these systems into outpatient interventional pain practices remains costly. This paper investigates a cost-effective Augmented Reality (AR) navigation system tailored for image-guided spinal pain procedures. We verify the feasibility of AR in spinal surgery and the potential of AR to replace fluoroscopy.
State-of-the-art computer-assisted surgical navigation devices are priced from $250,000 to $600,000 [1], posing a financial barrier for small healthcare providers. In this paper, we present a proof-of-concept investigation for a low-cost 3D navigation system utilizing Augmented Reality (AR). Our approach uses fiducial skin markers on the patient and Magnetic Resonance Imaging (MRI) of the region of surgical interest to generate a detailed 3D augmented reality anatomical representation. This virtual representation is then overlaid on the patient using fiducial skin markers for registration. Navigation systems of this type protect physicians and patients from the radiation associated with traditional imaging techniques like fluoroscopy [2] and reduce medical costs. This paper focuses on the applications of AR and tests the accuracy of an AR system in pain management surgery.
To achieve the goal of evaluating AR in back surgery, we first built a physical model simplifying the spine as a cylinder encased in soft material. We then created a 3D computer model of the physical spine using Computer-Aided Design techniques (CAD). Thereafter, we used real-time 3D AR software (Unity) and a commercially available Mixed Reality (MR) headset (Meta Quest 3 (retail price: $500.00), Meta Platforms) [3] to overlay the virtual spine on the physical spine model in which the spine is hidden by the mold. By analyzing the accuracy of simulated needle injection with and without the AR system, we determined the accuracy obtained with the AR approach.
Despite the high precision of fluoroscopy, incorporating AR technologies with MRI imaging and CAD modeling is advantageous due to radiation reduction and affordability. The integration of AR in healthcare has dramatically changed the landscape of surgical procedures. For example, Molina, et al. [4] have employed augmented reality during surgery to precisely identify bones and soft tissues in patients. To validate the proposed AR approach, we simulate a standard spinal needle injection procedure using a simplified spinal model.
The simplified spine model was manufactured by encasing a 3D-printed cylinder in a cube of ballistic gel (Figure 1a) [5]. The gel-covered cylinder (Figure 1b) represents the patient’s body. A metal detector was positioned at the center of the spine model (see point P) to provide feedback (LED light signal) if the surgical needle made contact with the detector. The detector was 3 mm in diameter, corresponding to a typical region of interest for surgeons during back surgery. Figure 1c shows the circuit design of the detection feedback system.
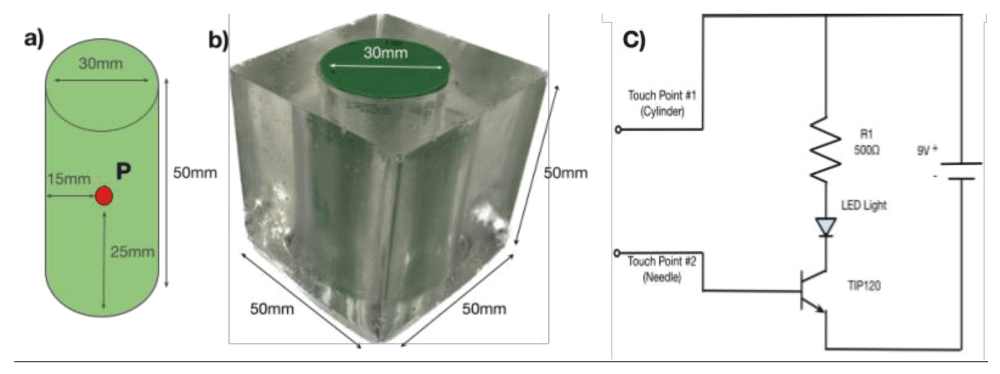 Figure 1: Simplified spinal model with a detection feedback circuit. a) Cylinder representing the spine. b) Cylinder encased in gel representing the body of the patient. c) Circuit for detecting contact.
Figure 1: Simplified spinal model with a detection feedback circuit. a) Cylinder representing the spine. b) Cylinder encased in gel representing the body of the patient. c) Circuit for detecting contact.We utilize a commercially available headset with MR support (Meta Quest 3 ($500.00), Meta Platforms) [3]. Using commercially available AR software (Unity) with specialized software packages, we created an environment that manages both AR and real objects. Since our cylindrical spine model was created from a CAD model, we were able to create a virtual model that is an exact replica of the physical spine model. This virtual model is viewable through the Meta Quest headset. Thus, instead of using fluoroscopy to view the spine, the surgeon wears a headset to see a virtual spine overlaid on the patient (physical model). This setup simulates the situation during surgery where doctors cannot see the spine through the skin.
To quantify the accuracy of the AR approach, we proceeded as follows. We first used our spine model (Figure 1) with the spine obscured by the gel and tried to place the surgical needle by visual guidance in the center of the model where the detector is located. After completing this baseline test, we superimposed AR imaging on the model and repeated the procedure guided by the virtual image. A total of 60 needle insertion trials were performed, 30 without the AR system and 30 with the use of the AR system. A trial was deemed successful when the needle touched the detector on our model, as confirmed by the LED lighting up. To determine the displacement distance of unsuccessful needle insertions, we measured the distance between the holes punctured by the needle on the cylinder and the center of the detector. In the experiment, all insertions made contact with the physical model. We define the success rate of insertion as:
And the average deviation as:
As depicted in Table 1, the use of the AR system increased the success rate by 40% as compared to the case without AR guidance. In addition, the average of the needle distance from the target for unsuccessful attempts was reduced by approximately 50%.
| Table 1: Experimental Success Rates and Average Deviations With VS Without AR System. | ||
| Without AR System | With AR System | |
| Success Rate | 50% | 70% |
| Average Deviation | 1.60cm | 0.83cm |
Our study shows the potential of the use of AR in spinal surgery as a safe and cost-effective alternative to fluoroscopy. In a real medical application, the key to the use of AR is the availability of the virtual spine that is overlaid on the patient and registered using fiducial markers. Since an MRI is a prerequisite for most spinal procedures, it is an easy step to create a 3D CAD model of the spine from MRI images. Incorporating fiducial markers in the MRI 3D display and registering the MRI virtual image of the fiducial markers on the patient, the AR system allows the surgeon to view the patient with all internal organs, blood vessels, and anatomical features visible. This is a great advantage compared to fluoroscopic procedures. With a $500 AR system and open-source modeling files, in contrast to professional fluoroscopy systems ranging from $100,000 to $500,000, our study shows the potential for significant cost savings. In addition, the use of AR eliminates radiation damage, ensuring a safer environment for both hospital staff and patients.
While our results with a $500 AR system and open-source modeling files are promising, further research is needed to achieve accuracy levels near 100%, as expected with fluoroscopy. One future consideration is the incorporation of Convolutional Neural Networks (CNNs) for the reconstruction of MRI models in AR applications. Research has shown that CNNs can reconstruct 3D models while simultaneously reducing image noise, potentially leading to greater accuracy in real-world scenarios [6,7]. One final note that must be mentioned is that our experiments did not account for real-world variables, such as patient-specific factors like breathing movements, sweating, and medical anomalies. These challenges must be addressed in future designs by reconstructing proper spinal models in CAD using advanced medical imaging techniques to enhance system robustness in operating room environments.
This study demonstrates the potential of low-cost AR applications in minimally invasive spinal surgeries. Using a simplified spinal model, a detection feedback system, and AR objects generated through CAD modeling, we assessed the accuracy of our navigation system. Our proposed $500 AR setup holds promise as an alternative to existing spinal surgery navigation systems. Future research will include the development and integration of trackable surgical instruments via computer vision techniques, thereby enabling surgeons to monitor the position of their medical equipment (scalpel, needle) relative to the patient’s bone anatomy in real time. Also, future efforts will include developing a more realistic spinal model and incorporating CNN algorithms to further enhance system precision. We expect our proposed tracking system to find application in minimally invasive surgeries at small to midsize healthcare facilities.
Malham GM, Wells-Quinn T. What should my hospital buy next?-Guidelines for the acquisition and application of imaging, navigation, and robotics for spine surgery. J Spine Surg. 2019 Mar;5(1):155-165. doi: 10.21037/jss.2019.02.04. PMID: 31032450; PMCID: PMC6465454.
Stahl CM, Cormier J, et al. Radiation risk to the fluoroscopy operator and staff. AJR Am J Roentgenol. 2016;207(4):737-744. PMID: 28829623.
Yang Z, Xu L, et al. Advances and challenges in microdisplays and imaging optics for virtual reality and mixed reality. Device. 2024;2(6):100139.
Molina CA, Theodore N, Ahmed AK, Westbroek EM, Mirovsky Y, Harel R, Orru' E, Khan M, Witham T, Sciubba DM. Augmented reality-assisted pedicle screw insertion: a cadaveric proof-of-concept study. J Neurosurg Spine. 2019 Mar 29;31(1):139-146. doi: 10.3171/2018.12.SPINE181142. PMID: 30925479.
Li W, Zhou Y, et al. Penetration of ballistic gelatin by explosion-driven inert metal particles. Latin Am J Solids Struct. 2024;21(3). ISSN: 1679-7825.
Alidoost F, Arefi H, Tombari F. 2D Image-to-3D Model: Knowledge-Based 3D Building Reconstruction (3DBR) Using Single Aerial Images and Convolutional Neural Networks (CNNs). Remote Sens. 2019;11(19).
Zavala-Mondragon LA, de With PHN, van der Sommen F. Image Noise Reduction Based on a Fixed Wavelet Frame and CNNs Applied to CT. IEEE Trans Image Process. 2021;30:9386-9401. doi: 10.1109/TIP.2021.3125489. Epub 2021 Nov 17. PMID: 34757905.
Lu S, Hui J, Lee E, Tsui D, Ahadian FA, Talke FR. Use of Augmented Reality as a Radiation-free Alternative in Pain Management Spinal Surgeries. IgMin Res. Aug 09, 2024; 2(8): 709-711. IgMin ID: igmin236; DOI:10.61927/igmin236; Available at: igmin.link/p236
次のリンクを共有した人は、このコンテンツを読むことができます:
1Center for Memory and Recording Research (CMRR), University of California, San Diego, USA
2School of Electrical and Computer Engineering, Georgia Institute of Technology, USA
3Department of Anesthesiology, Center for Pain Medicine, University of California, San Diego, USA
Address Correspondence:
Frank E Talke, Center for Memory and Recording Research (CMRR), University of California, San Diego, USA, Email: [email protected]
How to cite this article:
Lu S, Hui J, Lee E, Tsui D, Ahadian FA, Talke FR. Use of Augmented Reality as a Radiation-free Alternative in Pain Management Spinal Surgeries. IgMin Res. Aug 09, 2024; 2(8): 709-711. IgMin ID: igmin236; DOI:10.61927/igmin236; Available at: igmin.link/p236
Copyright: © 2024 Lu S, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
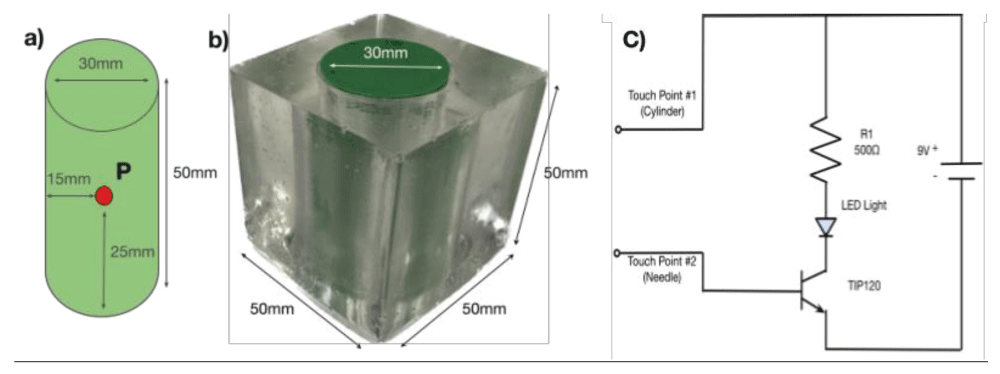 Figure 1: Simplified spinal model with a detection feedback ...
Figure 1: Simplified spinal model with a detection feedback ...
 Table 1: Experimental Success Rates and Average Deviations ...
Table 1: Experimental Success Rates and Average Deviations ...
Malham GM, Wells-Quinn T. What should my hospital buy next?-Guidelines for the acquisition and application of imaging, navigation, and robotics for spine surgery. J Spine Surg. 2019 Mar;5(1):155-165. doi: 10.21037/jss.2019.02.04. PMID: 31032450; PMCID: PMC6465454.
Stahl CM, Cormier J, et al. Radiation risk to the fluoroscopy operator and staff. AJR Am J Roentgenol. 2016;207(4):737-744. PMID: 28829623.
Yang Z, Xu L, et al. Advances and challenges in microdisplays and imaging optics for virtual reality and mixed reality. Device. 2024;2(6):100139.
Molina CA, Theodore N, Ahmed AK, Westbroek EM, Mirovsky Y, Harel R, Orru' E, Khan M, Witham T, Sciubba DM. Augmented reality-assisted pedicle screw insertion: a cadaveric proof-of-concept study. J Neurosurg Spine. 2019 Mar 29;31(1):139-146. doi: 10.3171/2018.12.SPINE181142. PMID: 30925479.
Li W, Zhou Y, et al. Penetration of ballistic gelatin by explosion-driven inert metal particles. Latin Am J Solids Struct. 2024;21(3). ISSN: 1679-7825.
Alidoost F, Arefi H, Tombari F. 2D Image-to-3D Model: Knowledge-Based 3D Building Reconstruction (3DBR) Using Single Aerial Images and Convolutional Neural Networks (CNNs). Remote Sens. 2019;11(19).
Zavala-Mondragon LA, de With PHN, van der Sommen F. Image Noise Reduction Based on a Fixed Wavelet Frame and CNNs Applied to CT. IEEE Trans Image Process. 2021;30:9386-9401. doi: 10.1109/TIP.2021.3125489. Epub 2021 Nov 17. PMID: 34757905.