
Study of the Histological Features of the Stroma of High-Grade Gliomas Depending on the Status of the Mutation in the IDH1 Gene
Pathology NeurologyOncologyParasitology受け取った 30 Jul 2024 受け入れられた 06 Aug 2024 オンラインで公開された 07 Aug 2024
Focusing on Biology, Medicine and Engineering ISSN: 2995-8067 | Quick Google Scholar
Next Full Text
Use of Augmented Reality as a Radiation-free Alternative in Pain Management Spinal Surgeries
Previous Full Text
Mastocytosis: Principles and Pitfalls in the Diagnosis of a Unique Disease


受け取った 30 Jul 2024 受け入れられた 06 Aug 2024 オンラインで公開された 07 Aug 2024
High-grade gliomas are known for their aggressive nature and resistance to therapy. One characteristic feature of these tumors is the lack of a clear border between the tumor and normal brain tissue. Previous studies have shown that as gliomas dedifferentiate, the extracellular matrix (ECM) undergoes changes in its composition and architecture. This is due to increased production and overexpression of ECM components such as hyaluronic acid, fibulin-3, and collagen. However, it is not yet known what specific changes occur in the stroma of high-grade gliomas depending on the v in the IDH1 gene. In our study, we examined tumor tissue samples from 31 patients, 10 of whom had verified IDH-mutant astrocytoma (grade 4) and 21 had IDH-wildtype glioblastoma (grade 4). The presence or absence of mutations in the IDH1/2 genes was determined in all patients using immunohistochemistry (IHC) and polymerase chain reaction (PCR). To assess stromal changes, we used histochemical staining with Alcian blue and Mallory trichrome. Our results showed significant differences between the two groups according to Student’s t-test (p < 0.05) for all stainings. The presence of mucus formation, collagen formation, and expression of vimentin by tumor cells in the stroma of IDH-wildtype grade 4 glioblastoma indicates an active epithelial-mesenchymal transition and changes in the extracellular matrix. These findings may explain the more unfavorable prognosis in patients with glioblastomas and could potentially serve as a therapeutic target in the complex treatment of malignant gliomas.
Malignant gliomas are aggressive tumors of the Central Nervous System (CNS) that are known for their diffuse-infiltrative growth and resistance to therapy []. One defining characteristic of high-grade gliomas is the lack of a clear boundary between the tumor and normal tissue, as tumor cells can infiltrate the brain parenchyma at a significant distance from the original tumor node. In Russia, the incidence of malignant gliomas is estimated to be 5-8 cases per 100,000 population [], with high-grade gliomas accounting for approximately 70% of all malignant CNS tumors [,].
Currently, the average survival time for patients diagnosed with glioblastoma is 14 months, taking into account surgery, radiation therapy, and chemotherapy []. However, radical surgical treatment for tumor removal can increase the risk of neurological disorders, negatively impacting the patient’s quality of life. In fact, patients who undergo exclusively surgical treatment have a survival time of no more than 6 months [,], while those who receive complex treatment have a survival time of only 15 months []. Despite advancements in glioma diagnosis, the prognosis for patients remains unfavorable. Therefore, special approaches to diagnosis and treatment are necessary for malignant gliomas.
While glioblastoma is not typically considered a metastatic tumor in the traditional sense, with metastasis rates ranging from 0.2% to 2% [], it can still spread locally and invade surrounding brain tissue. Inhibiting cell migration and invasion may be beneficial in limiting the tumor’s spread and invasive behavior within the brain. It is well-known that glioblastoma is characterized by the development of an abundant pathological vascular network, which not only promotes tumor growth but also contributes to its high rate of invasion into adjacent brain tissue [,]. However, there is a lack of comprehensive descriptions of changes in the tumor stroma in the literature.
Since 2007, the World Health Organization (WHO) has recognized the impact of mutations in the IDH1 and 2 genes on the prognosis of glial tumors in their classifications of CNS tumors. However, it was not until the 2021 WHO classification of CNS tumors that glial tumors were strictly divided into wild-type (ICD-O code 9440/3) and mutant (ICD-O code 9445/3) tumors based on gene status [,]. The IDH1 gene produces the enzyme isocitrate dehydrogenase 1, which plays a role in regulating cellular redox status by catalyzing the reversible oxidative decarboxylation of isocitrate to form alpha-ketoglutarate. Mutations in the IDH1 gene typically affect amino acid residue 132, which is part of the active center. The majority (>85%) of these mutations are heterozygous missense mutations, with the most common being the replacement of arginine with histidine (R132H) []. The mutated IDH1 protein converts alpha-ketoglutarate into 2-hydroxyglutarate, which is considered an oncometabolite [,]. Therefore, it is possible that changes in the tricarboxylic acid cycle in glial tumor cells, caused by a mutation in the IDH1 gene, may lead to alterations in the stromal component of high-grade gliomas.
To investigate the histological characteristics of the stroma in high-grade gliomas, with consideration of the IDH1 gene mutation status.
We conducted a retrospective study on biopsy samples of tumor fragments. These samples were obtained intraoperatively from 31 patients, 10 of whom were diagnosed with IDH-mutant astrocytoma grade 4, and 21 with IDH-wildtype glioblastoma grade 4. The IDH-mutant astrocytoma group (IDH-mut AC) consisted of 3 women and 7 men, aged 23 to 65 years, with an average age of 38.5 years. The IDH-wildtype glioblastoma group (IDH-wildtype GB) consisted of 10 women and 11 men, aged 38 to 82 years, with an average age of 60 years. The samples were fixed in 10% buffered formalin, dehydrated, and embedded in paraffin using standard procedures. Histological sections were stained with hematoxylin and eosin, and immunohistochemical (IHC) reactions were performed using antibodies to IDH1R132h and vimentin (from Diagnostic Biosystems, USA) with the EnVision visualization system. Histochemical staining with alcian blue and Mallory trichrome (BioVitrum, Russia) was also conducted. To confirm the presence or absence of mutations in the IDH1/2 genes, Polymerase Chain Reaction (PCR) was performed on all patients using the TestGene kit (Russia). Histological analysis and microphotography were carried out using a Leica Aperio AT2 scanning microscope and AperioImageScope image manager (Leica Microsystems, USA). The results of histochemical staining and immunohistochemical reaction with antibodies to vimentin were evaluated by quantifying the number of pixels corresponding to a positive reaction in the tumor stroma using the author’s software in 1 mm2 of tissue in the Python programming language. Statistical analysis was performed using the NumPy, SciPy, Pandas, and Matplotlib libraries in Python. The Shapiro-Wilk test was used to assess the normality of the data distribution. Indicators with a normal distribution were reported as the mean value and standard deviation. Since the data followed a normal distribution, parametric statistical methods, specifically Student’s t-test, were used to compare indicators between groups. Spearman’s correlation method was used to identify any correlations, with the strength of the relationship interpreted according to the Chaddock scale. Differences were considered statistically significant if the p - value was less than 0.05. The study was conducted in accordance with the Helsinki Declaration of Human Rights. All patients (their representatives) signed informed voluntary consent to participate in the study. Preoperative examination and surgical treatment of patients were carried out in accordance with the Clinical Guidelines of the Association of Neurosurgeons of Russia 2015.
The morphological picture of a malignant glial tumor was verified in all patients when studying the histological material. When conducting IHC reactions with an antibody to IDH1R132h in patients with IDH-mutant grade 4 astrocytoma, positive staining was detected, and the presence of mutations in the IDH1 gene was confirmed by PCR. In patients with IDH-wildtype grade 4 glioblastoma, when conducting IHC with an antibody to IDH1R132h, staining was absent, and the absence of mutations in the IDH1 and IDH2 genes was confirmed by PCR. When conducting IHC reactions with vimentin, its bright diffuse immunopositivity of the cytoplasm of tumor cells in glioblastoma IDH-wildtype grade 4 (GB IDH-wt) was revealed, while in astrocytoma IDH-mutant grade 4 (AC IDH-mut) expression was detected in single cells and vessel walls (Figure 1).
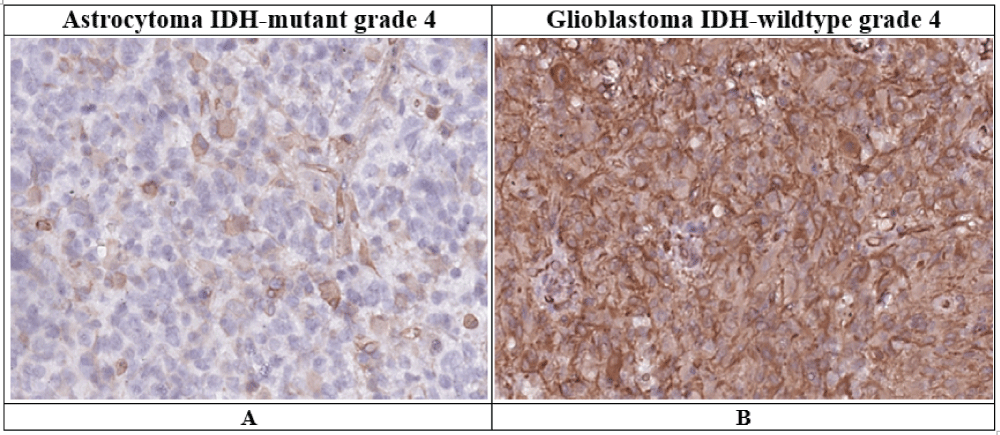 Figure 1: Vimentin expression in grade 4 astrocytoma IDH-mutant and IDH-wildtype glioblastoma (description in text).Immunohistochemical reaction, ×400A – Astrocytoma IDH-mutant grade 4.B – Glioblastoma IDH-wildtype grade 4.
Figure 1: Vimentin expression in grade 4 astrocytoma IDH-mutant and IDH-wildtype glioblastoma (description in text).Immunohistochemical reaction, ×400A – Astrocytoma IDH-mutant grade 4.B – Glioblastoma IDH-wildtype grade 4.Histochemical staining with Alcian blue showed that most AC IDH-mut did not have any staining in the tumor stroma but did show a positive reaction in the vessels. In contrast, the stroma of many GB IDH-wt samples exhibited significant “mucinization” and a bright blue staining (Figure 2A,C). Histochemical staining with Mallory trichrome revealed that the “fibrous” component of the stroma was largely absent in AC IDH-mut, while IDH-wildtype GBs showed varying degrees of collagen formation (Figure 2B,D). Additionally, Mallory staining revealed fibrinoid necrosis in isolated large-caliber tumor vessels.
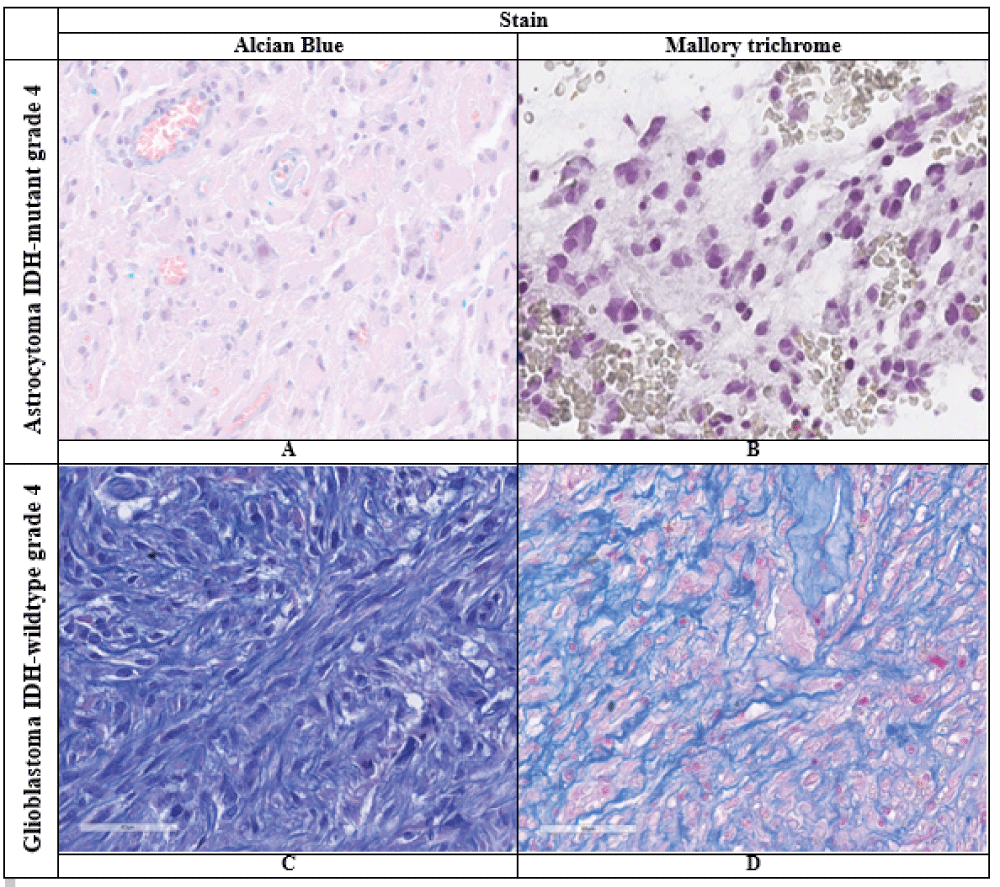 Figure 2: Results of histochemical reactions in the studied tumors (description in the text), ×400. A, C – Alcian blue stain; B, D – Mallory trichrome stain.
Figure 2: Results of histochemical reactions in the studied tumors (description in the text), ×400. A, C – Alcian blue stain; B, D – Mallory trichrome stain.The results from counting the number of pixels with a positive reaction in the cell cytoplasm (measured in conventional units, c.u.) in 1 mm2 of tumor tissue, as well as the statistical data analysis, are presented in Table 1. Our findings indicate that the expression of vimentin in patients with grade 4 gliomas varies significantly depending on the presence or absence of a mutation in the IDH gene, as determined by Student’s t-test (p < 0.05) (Table 1). Additionally, there was a significant difference between the groups in terms of staining with Alcian blue and Mallory trichrome (p < 0.05) (Table 1).
 Table 1: Results of statistical data processing for the studied stains in IDH-mutant grade 4 astrocytoma and IDH-wildtype glioblastoma grade 4, p-value result (Student's t-test).
Table 1: Results of statistical data processing for the studied stains in IDH-mutant grade 4 astrocytoma and IDH-wildtype glioblastoma grade 4, p-value result (Student's t-test).The correlation analysis results for vimentin expression and tumor stroma staining in grade 4 glioma patients are presented in Table 2. In IDH-wt GB, a strong positive correlation (Spearman correlation coefficient of 0.87) was found between Mallory trichrome and Alcian blue staining. Additionally, a moderate positive correlation (Spearman correlation coefficient of 0.61) was observed between vimentin expression and Alcian blue staining. However, the remaining results showed a weak relationship and can be disregarded.
 Table 2: Correlation analysis between vimentin expression level, alcian blue, Mallory trichrome stains in astrocytoma IDH-mutant grade 4 (red) / glioblastoma IDH-wildtype grade 4 (blue) (description in the text).
Table 2: Correlation analysis between vimentin expression level, alcian blue, Mallory trichrome stains in astrocytoma IDH-mutant grade 4 (red) / glioblastoma IDH-wildtype grade 4 (blue) (description in the text).High-grade gliomas are known for their aggressive nature and resistance to therapy []. The interaction of tumor cells with each other and with other cells during carcinogenesis contributes to the formation of a more metabolically efficient microenvironment, which is no exception for malignant gliomas []. There are two signaling pathways of influence: autocrine and paracrine []. Substances released during the interaction of microenvironment cells with each other and with tumor cells act as chemoattractants for various types of cells, including fibroblasts, smooth muscle cells, endothelial cells, neutrophilic and eosinophilic leukocytes, basophils, mast cells, NK cells, T and B lymphocytes, macrophages, and dendritic cells. These substances also play a role in changing the intercellular substance and promoting neovascularization processes [,]. Additionally, high levels of CD13 expression have been linked to poor survival and prognosis in glioblastoma patients and are positively associated with clinical malignant characteristics [].
Recent studies have shown that glioma stem cells (GSCs) play a significant role in therapy resistance and tumor recurrence []. The Cancer Genome Atlas has identified four molecular subtypes of IDH-wildtype GB (classical, mesenchymal (MES), neural, and proneural), each with different disease prognosis and response rates to chemotherapy [-]. The MES subtype is particularly aggressive and resistant to radiation [,]. Research has also shown that the acquisition of the MES phenotype contributes to the malignant characteristics and tumorigenicity of GSCs. In a study by Song, et al, increased expression of Salmonella pathogenicity island 1 (SPI1) was found to be positively correlated with the MES phenotype, which in turn was associated with poor survival. In vitro experiments demonstrated that SPI1 promotes MES GSC differentiation, self-renewal, and radioresistance, while also promoting tumorigenesis in vivo. Mechanistically, SPI1 increased the transcriptional activity of both transforming growth factor-β1 (TGF-β1) and FKBP12, activating the non-canonical PI3K/Akt pathway. Notably, inhibition of TGF-β1/PI3K/Akt sissgnaling partially attenuated SPI1-induced MES GSC differentiation and the associated malignant phenotype [].
Despite the use of a multimodal approach in treating malignant gliomas, the main reason for therapeutic failure is the intrinsic intratumoral heterogeneity, which is largely influenced by the complexity of the Tumor Microenvironment (TME) and its ability to evade the immune system []. The TME consists of various differentiated tumor cell populations, stem cells, neuronal cells, and both resident and infiltrating immune cells, all of which are encapsulated by the Extracellular Matrix (ECM). The ECM is a complex network of glycosaminoglycans, glycoproteins, and proteoglycans that exist in both healthy tissues and malignancies. As glioma progresses to GBM, the composition and architecture of the ECM undergo remodeling due to increased production and overexpression of components such as hyaluronic acid (HA), fibulin-3, and collagen []. In our study, we observed increased collagen synthesis in IDH-wt GB when comparing Mallory staining results, while collagen fibers were virtually absent in IDH-mut ACs. Additionally, we found mucoid changes in Alcian blue staining in IDH-wt GB, but minimal stromal changes in IDH-mut AC. This overexpression of ECM creates a protective barrier around the tumor, limiting the diffusion of immune system components and drugs into the tumor and ultimately reducing therapeutic efficacy. However, it also presents potential targets for new therapeutic agents [,].
Epithelial-Mesenchymal Transition (EMT) is closely associated with the malignant progression and clinical outcome of gliomas []. During EMT, epithelial cells acquire a mesenchymal phenotype and properties, including migration. This process is crucial in the formation of metastatic foci, as cancer cells gain the ability to migrate to distant sites from the primary tumor. Additionally, EMT has been linked to drug resistance in gliomas. Several mechanisms have been identified as contributors to glioma EMT. However, the induction of these processes in glioblastomas differs from that observed in epithelial cancers due to the absence of a basement membrane []. In typical cases, tumor cells must detach from the basement membrane to migrate to other parts of the body. However, in the case of gliomas, glial cells are the most abundant cells in the brain and provide protective and structural support []. Astrocytes play a crucial role in the development and progression of brain tumors. They are activated and surround the tumor stroma, providing a protective barrier that keeps cancer cells and the cluster intact. However, cancer cells can induce EMT processes in astrocytes, leading to aberrant signaling pathways that promote migration and metastasis []. On the other hand, astrocytes also have a protective function, secreting molecules that maintain the integrity of the blood-brain barrier and prevent cancer cell invasion. It is worth noting that the existing literature mainly covers EMT in IDH-wt astrocytes, while the processes in IDH-mut astrocytes have not been extensively studied. In our study, we observed vimentin expression, a marker of EMT, only in a small number of IDH-mut astrocytes, while it was consistently strong in IDH-wt astrocytes. This finding is consistent with the study by Shi, et al, which showed that TGF-β1 induces EMT in U87 and U251 cells, resulting in a decrease in the epithelial marker ZO-1 and an increase in the mesenchymal markers N-cadherin and vimentin [,]. Furthermore, the study by Xu, et al. examined the impact of tubulin polymerization promoting protein 3 (TPPP3) on gliomas and revealed that TPPP3 expression was significantly higher in glioma tissue compared to normal brain tissue, with levels increasing as the grade of glioma increased. The researchers also found that upregulation of TPPP3 in glioblastoma cells led to increased migration, invasion, and proliferation abilities, as well as decreased apoptosis in vitro. Additionally, TPPP3 was found to influence the process of epithelial-mesenchymal transition (EMT) by regulating the expression of Snail 1 protein [].
Several studies have demonstrated that glioma-associated microglia/macrophages (GAM) play a significant role in promoting glioma progression [,]. For instance, one study proposed an axis involving CCL2/CCR2/interleukin-6 between glioma cells and GAM, which may contribute to tumor invasiveness []. Previous research has also shown that GAM can release factors such as epidermal growth factor (EGF) and transforming growth factor-β (TGF-β) to enhance glioma cell migration and growth []. In a study by He, et al, it was found that GAM not only has a strong association with gliomas and their malignancy but also has a close correlation with the degree of epithelial-mesenchymal transition (EMT) in gliomas. Furthermore, the researchers isolated GAMs from glioma samples and used co-culture models (in vitro) to demonstrate the role of GAMs in stimulating the EMT process in glioma cells, providing evidence for the oncogenic effects of GAMs in EMT in gliomas [43].
The stroma of IDH-wildtype grade 4 glioblastoma is characterized by the pronounced formation of mucus, collagen, and vimentin expression, unlike IDH-mutant grade 4 astrocytoma. These changes indicate an active epithelial-mesenchymal transition and alterations in the extracellular matrix. These factors may contribute to the more unfavorable prognosis in patients with IDH-wildtype grade 4 GB and could potentially serve as a therapeutic target in the comprehensive treatment of malignant gliomas.
We thank the staff of Polenov Neurosurgical Institute – Branch of Almazov National Medical Research Centre who treat patients with brain tumours. The authors express special gratitude to Nina A. Grosheva and Sofia O. Cobeleva for their highly professional preparation of microscopic specimens.
The study was performed without external funding.
Compliance with patient rights and principles of bioethics. All patients gave written informed consent to participate in the study.
Choinzonov YeL, Gribova OV, Startseva ZhA, Ryabova AI. Current approaches to chemoradiotherapy for malignant gliomas. Bulletin of Siberian Medicine. 2014;13(3):119-125.
Bliseeva АV. Cancer in Russia in 2009 (morbidity and mortality). Мoscow. 2010;17.
Mufazalov FF, Abbasova RR, Mufazalova NA, Goncharova OV. Modern tactics of treatment of malignant glyom of the brain and the case of the complete answer of the tumor on the background of the long-term reception of bevacizumab. Malignant tumours. 2017;(2):33-39.
Sklyar S, Sitovskaya D, Mirolyubova Y, Kushnirova V. Lymphopenia in Patients with Glioblastoma: Literature Review and Case Presentations. Journal of Clinical Surgery and Surgical Research, BioRes Scientia Publishers. 2024;3(3):1-8.
McGirt MJ, Mukherjee D, Chaichana KL, Than KD, Weingart JD, Quinones-Hinojosa A. Association of surgically acquired motor and language deficits on overall survival after resection of glioblastoma multiforme. Neurosurgery. 2009 Sep;65(3):463-9; discussion 469-70. doi: 10.1227/01.NEU.0000349763.42238.E9. PMID: 19687690.
Rynda AY, Olyushin VE, Rostovtsev DM, Zabrodskaya YM, Ulitin AY, Papayan GV. Intraoperative photodynamic therapy in complex treatment of malignant gliomas. Zh Vopr Neirokhir Im N N Burdenko. 2023;87(1):25-34. English, Russian. doi: 10.17116/neiro20238701125. PMID: 36763550.
Ferrés A, Di Somma A, Mosteiro A, Topczewski TE, Roldán P, Pedrosa L, Diao D, Pineda E, Sierra À, Enseñat J, González-Sánchez JJ. Photodynamic therapy in glioblastoma: Detection of intraoperative inadvertent 5-ALA mediated photodynamic therapeutical effect after gross total resection. Front Oncol. 2022 Dec 2;12:1080685. doi: 10.3389/fonc.2022.1080685. PMID: 36531012; PMCID: PMC9755173.
Stupp R, Hegi ME, Mason WP, van den Bent MJ. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459-66.
Strong MJ, Koduri S, Allison JA, Pesavento CM, Ogunsola S, Ogunsola O, Yee TJ, Khalsa SSS, Saadeh YS, Joseph JR, Kashlan ON, Park P, Oppenlander ME, Szerlip NJ. Bone metastasis from glioblastoma: a systematic review. J Neurooncol. 2022 Jul;158(3):379-392. doi: 10.1007/s11060-022-04025-4. Epub 2022 May 17. PMID: 35578056.
Kim AV, Khachatryan VA, Samochernykh KA. Ekstranevral’noe metastazirovanie glioblastomy. Vestnik khirurgii imeni I. I. Grekova. 2007;166 (6), 70–74.
Awan M, Liu S, Sahgal A, Das S, Chao ST, Chang EL, Knisely JP, Redmond K, Sohn JW, Machtay M, Sloan AE, Mansur DB, Rogers LR, Lo SS. Extra-CNS metastasis from glioblastoma: a rare clinical entity. Expert Rev Anticancer Ther. 2015 May;15(5):545-52. doi: 10.1586/14737140.2015.1028374. PMID: 25907706.
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, Soffietti R, von Deimling A, Ellison DW. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol. 2021 Aug 2;23(8):1231-1251. doi: 10.1093/neuonc/noab106. PMID: 34185076; PMCID: PMC8328013.Trashkov AP, Spirin AL, Tsygan NV, Artyomenko MR. Cerebral Glial Tumors: General Principles of Diagnostics and Treatment // Pediatrician (St. Petersburg). 2015;6(4):75-84.
Watanabe T, Vital A, Nobusawa S, Kleihues P, Ohgaki H. Selective acquisition of IDH1 R132C mutations in astrocytomas associated with Li-Fraumeni syndrome. Acta Neuropathol. 2009 Jun;117(6):653-6. doi: 10.1007/s00401-009-0528-x. Epub 2009 Apr 2. PMID: 19340432.
Jin G, Reitman ZJ, Spasojevic I, Batinic-Haberle I, Yang J, Schmidt-Kittler O, Bigner DD, Yan H. 2-hydroxyglutarate production, but not dominant negative function, is conferred by glioma-derived NADP-dependent isocitrate dehydrogenase mutations. PLoS One. 2011 Feb 4;6(2):e16812. doi: 10.1371/journal.pone.0016812. PMID: 21326614; PMCID: PMC3033901.
Garber K. Oncometabolite? IDH1 discoveries raise possibility of new metabolism targets in brain cancers and leukemia. J Natl Cancer Inst. 2010 Jul 7;102(13):926-8. doi: 10.1093/jnci/djq262. Epub 2010 Jun 24. PMID: 20576929.
Nayak L, Reardon DA. High-grade Gliomas. Continuum (Minneap Minn). 2017 Dec;23(6, Neuro-oncology):1548-1563. doi: 10.1212/CON.0000000000000554. PMID: 29200110.
Wilke CM, Wu K, Zhao E, Wang G, Zou W. Prognostic significance of regulatory T cells in tumor. Int J Cancer. 2010 Aug 15;127(4):748-58. doi: 10.1002/ijc.25464. PMID: 20473951.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011 Mar 4;144(5):646-74. doi: 10.1016/j.cell.2011.02.013. PMID: 21376230.
Al-Zoughbi W, Al-Zhoughbi W, Huang J, Paramasivan GS, Till H, Pichler M, Guertl-Lackner B, Hoefler G. Tumor macroenvironment and metabolism. Semin Oncol. 2014 Apr;41(2):281-95. doi: 10.1053/j.seminoncol.2014.02.005. Epub 2014 Mar 1. Erratum in: Semin Oncol. 2014 Aug;41(4):e31. doi: 10.1053/j.seminoncol.2014.07.005. PMID: 24787299; PMCID: PMC4012137.
Sklyar SS, Sitovskaya DA, Mirolyubova IuV, Kushnirova VS. Immune system dysfunction in patients with glioblastoma. Literature review. Clinical cases. Rossiiskii neirokhirurgicheskii zhurnal imeni professora A. L. Polenova. 2023;15(4):200-208.
Zhang W, Blank A, Kremenetskaia I, Nitzsche A. CD13 expression affects glioma patient survival and influences key functions of human glioblastoma cell lines in vitro. BMC Cancer. 2024;22;24(1):369.
Mao P, Joshi K, Li J, Kim SH, Li P, Santana-Santos L, Luthra S, Chandran UR, Benos PV, Smith L, Wang M, Hu B, Cheng SY, Sobol RW, Nakano I. Mesenchymal glioma stem cells are maintained by activated glycolytic metabolism involving aldehyde dehydrogenase 1A3. Proc Natl Acad Sci U S A. 2013 May 21;110(21):8644-9. doi: 10.1073/pnas.1221478110. Epub 2013 May 6. PMID: 23650391; PMCID: PMC3666732.
Bhat KPL, Balasubramaniyan V, Vaillant B, Ezhilarasan R, Hummelink K, Hollingsworth F, Wani K, Heathcock L, James JD, Goodman LD, Conroy S, Long L, Lelic N, Wang S, Gumin J, Raj D, Kodama Y, Raghunathan A, Olar A, Joshi K, Pelloski CE, Heimberger A, Kim SH, Cahill DP, Rao G, Den Dunnen WFA, Boddeke HWGM, Phillips HS, Nakano I, Lang FF, Colman H, Sulman EP, Aldape K. Mesenchymal differentiation mediated by NF-κB promotes radiation resistance in glioblastoma. Cancer Cell. 2013 Sep 9;24(3):331-46. doi: 10.1016/j.ccr.2013.08.001. Epub 2013 Aug 29. PMID: 23993863; PMCID: PMC3817560.
Hu B, Ruan Y, Wei F, Qin G, Mo X, Wang X, Zou D. Identification of three glioblastoma subtypes and a six-gene prognostic risk index based on the expression of growth factors and cytokines. Am J Transl Res. 2020 Aug 15;12(8):4669-4682. PMID: 32913540; PMCID: PMC7476164.
Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, Misra A, Nigro JM, Colman H, Soroceanu L, Williams PM, Modrusan Z, Feuerstein BG, Aldape K. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006 Mar;9(3):157-73. doi: 10.1016/j.ccr.2006.02.019. PMID: 16530701.
Song Y, Zhang Y, Wang X, Han X, Shi M, Xu L, Yu J, Zhang L, Han S. SPI1 activates TGF-β1/PI3K/Akt signaling through transcriptional upregulation of FKBP12 to support the mesenchymal phenotype of glioma stem cells. Brain Pathol. 2024 May;34(3):e13217. doi: 10.1111/bpa.13217. Epub 2023 Oct 22. PMID: 37865975; PMCID: PMC11007049.
Prager BC, Bhargava S, Mahadev V, Hubert CG, Rich JN. Glioblastoma Stem Cells: Driving Resilience through Chaos. Trends Cancer. 2020 Mar;6(3):223-235. doi: 10.1016/j.trecan.2020.01.009. Epub 2020 Feb 3. PMID: 32101725; PMCID: PMC8779821.
Hambardzumyan D, Bergers G. Glioblastoma: Defining Tumor Niches. Trends Cancer. 2015 Dec;1(4):252-265. doi: 10.1016/j.trecan.2015.10.009. PMID: 27088132; PMCID: PMC4831073.
Mohiuddin E, Wakimoto H. Extracellular matrix in glioblastoma: opportunities for emerging therapeutic approaches. Am J Cancer Res. 2021 Aug 15;11(8):3742-3754. PMID: 34522446; PMCID: PMC8414390.
Giles B, Nakhjavani M, Wiesa A, Knight T. Unravelling the Glioblastoma Tumour Microenvironment: Can Aptamer Targeted Delivery Become Successful in Treating Brain Cancers? Cancers (Basel). 2023;1;15(17):4376.
Zhang Y, Zeng A, Liu S, Li R, Wang X, Yan W, Li H, You Y. Genome-wide identification of epithelial-mesenchymal transition-associated microRNAs reveals novel targets for glioblastoma therapy. Oncol Lett. 2018 May;15(5):7625-7630. doi: 10.3892/ol.2018.8280. Epub 2018 Mar 15. PMID: 29740486; PMCID: PMC5934713.
Iwadate Y. Epithelial-mesenchymal transition in glioblastoma progression. Oncol Lett. 2016 Mar;11(3):1615-1620. doi: 10.3892/ol.2016.4113. Epub 2016 Jan 14. PMID: 26998052; PMCID: PMC4774466.
Jäkel S, Dimou L. Glial Cells and Their Function in the Adult Brain: A Journey through the History of Their Ablation. Front Cell Neurosci. 2017 Feb 13;11:24. doi: 10.3389/fncel.2017.00024. PMID: 28243193; PMCID: PMC5303749.
Iser IC, Lenz G, Wink MR. EMT-like process in glioblastomas and reactive astrocytes. Neurochem Int. 2019 Jan;122:139-143. doi: 10.1016/j.neuint.2018.11.016. Epub 2018 Nov 26. PMID: 30496766.
Zhang H, Zhou Y, Cui B, Liu Z, Shen H. Novel insights into astrocyte-mediated signaling of proliferation, invasion and tumor immune microenvironment in glioblastoma. Biomed Pharmacother. 2020 Jun;126:110086. doi: 10.1016/j.biopha.2020.110086. Epub 2020 Mar 12. PMID: 32172060.
Shi L, Wang Z, Rong J, Fei X, Li X, He B, Gong W, Qian J. Inhibition of TGF-β1-induced epithelial-mesenchymal transition in gliomas by DMC-HA. Aging (Albany NY). 2023 Dec 27;15(24):15183-15195. doi: 10.18632/aging.205340. Epub 2023 Dec 27. PMID: 38154100; PMCID: PMC10781457.
Xu X, Hou Y, Long N, Jiang L, Yan Z, Xu Y, Lv Y, Xiang X, Yang H, Liu J, Qi X, Chu L. TPPP3 promote epithelial-mesenchymal transition via Snail1 in glioblastoma. Sci Rep. 2023 Oct 20;13(1):17960. doi: 10.1038/s41598-023-45233-w. PMID: 37863960; PMCID: PMC10589222.
Hambardzumyan D, Gutmann DH, Kettenmann H. The role of microglia and macrophages in glioma maintenance and progression. Nat Neurosci. 2016;19(1):20-7.
Coniglio SJ, Eugenin E, Dobrenis K, Stanley ER, West BL, Symons MH, Segall JE. Microglial stimulation of glioblastoma invasion involves epidermal growth factor receptor (EGFR) and colony stimulating factor 1 receptor (CSF-1R) signaling. Mol Med. 2012 May 9;18(1):519-27. doi: 10.2119/molmed.2011.00217. PMID: 22294205; PMCID: PMC3356419.
Zhang J, Sarkar S, Cua R, Zhou Y, Hader W, Yong VW. A dialog between glioma and microglia that promotes tumor invasiveness through the CCL2/CCR2/interleukin-6 axis. Carcinogenesis. 2012 Feb;33(2):312-9. doi: 10.1093/carcin/bgr289. Epub 2011 Dec 8. PMID: 22159219.
Wick W, Platten M, Weller M. Glioma cell invasion: regulation of metalloproteinase activity by TGF-beta. J Neurooncol. 2001 Jun;53(2):177-85. doi: 10.1023/a:1012209518843. PMID: 11716069.
He X, Guo Y, Yu C, Zhang H, Wang S. Epithelial-mesenchymal transition is the main way in which glioma-associated microglia/macrophages promote glioma progression. Front Immunol. 2023 Mar 10;14:1097880. doi: 10.3389/fimmu.2023.1097880. PMID: 36969175; PMCID: PMC10036378.
Sitovskaya D, Frolkova K, Shanina E, Sokolova T, Zabrodskaya Y. Study of the Histological Features of the Stroma of High-Grade Gliomas Depending on the Status of the Mutation in the IDH1 Gene. IgMin Res. August 07, 2024; 2(8): 702-0. IgMin ID: igmin235; DOI: 10.61927/igmin235; Available at: igmin.link/p235
次のリンクを共有した人は、このコンテンツを読むことができます:
1Almazov National Medical Research Centre, St. Petersburg, Russia
2Department of Pathology, Forensic Medicine named after D.D. Lochov, St. Petersburg State Pediatric Medical University, Saint-Petersburg, Russia
3Department of Pathology, Mechnikov North-West State Medical University, St. Petersburg, Russia
Address Correspondence:
Darya Sitovskaya, Department of Pathology, Forensic Medicine named after D.D. Lochov, St. Petersburg State Pediatric Medical University, Saint-Petersburg, Russia, Email: [email protected]
How to cite this article:
Sitovskaya D, Frolkova K, Shanina E, Sokolova T, Zabrodskaya Y. Study of the Histological Features of the Stroma of High-Grade Gliomas Depending on the Status of the Mutation in the IDH1 Gene. IgMin Res. August 07, 2024; 2(8): 702-0. IgMin ID: igmin235; DOI: 10.61927/igmin235; Available at: igmin.link/p235
Copyright: © 2024 Sitovskaya D, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
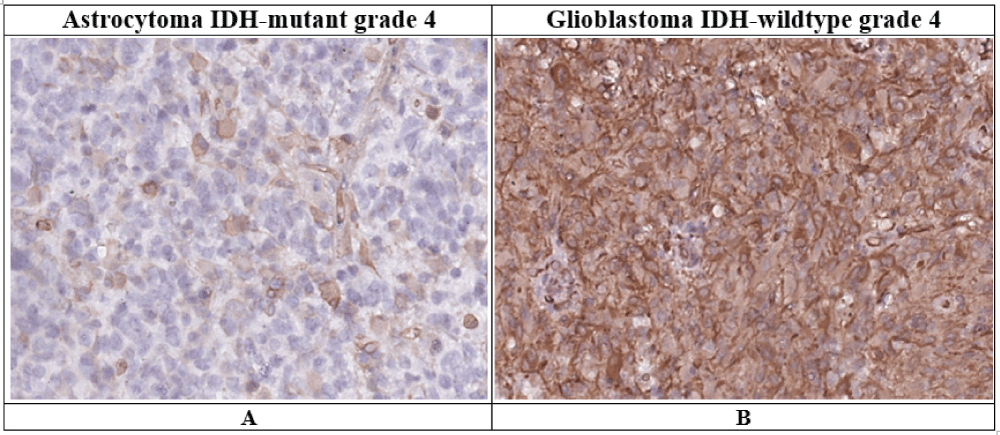 Figure 1: Vimentin expression in grade 4 astrocytoma IDH-mut...
Figure 1: Vimentin expression in grade 4 astrocytoma IDH-mut...
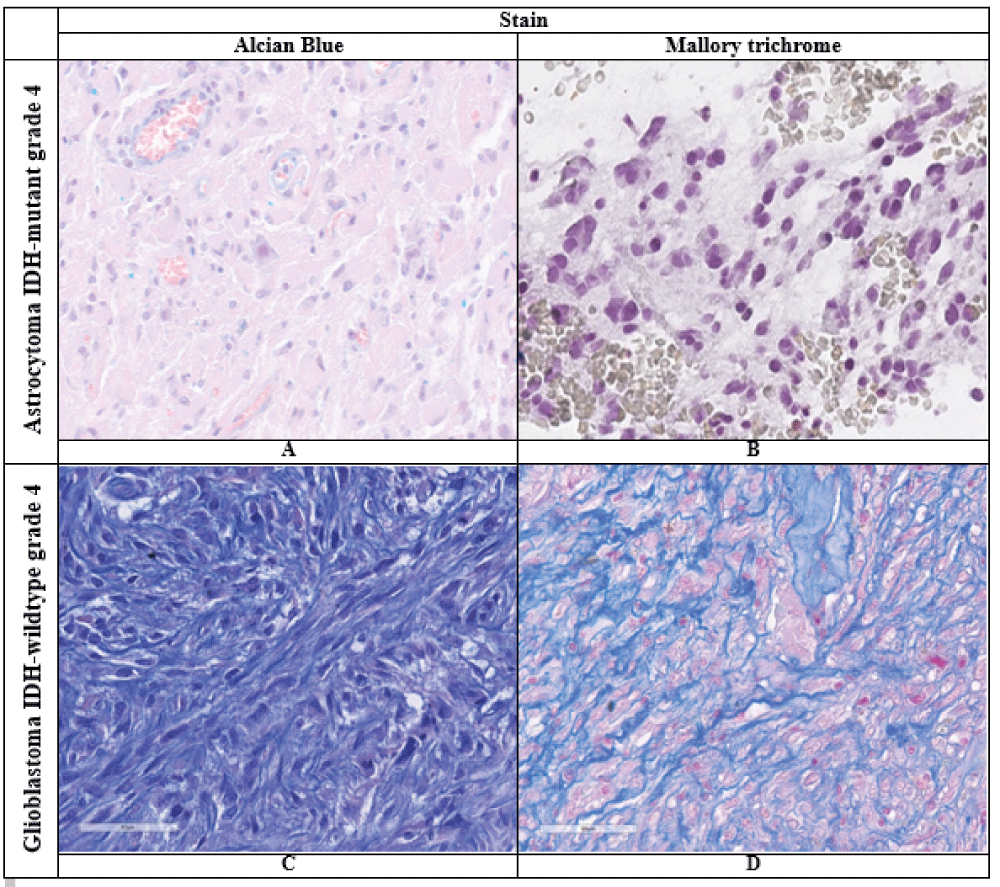 Figure 2: Results of histochemical reactions in the studied ...
Figure 2: Results of histochemical reactions in the studied ...
 Table 1: Results of statistical data processing for the stu...
Table 1: Results of statistical data processing for the stu...
 Table 2: Correlation analysis between vimentin expression l...
Table 2: Correlation analysis between vimentin expression l...
Choinzonov YeL, Gribova OV, Startseva ZhA, Ryabova AI. Current approaches to chemoradiotherapy for malignant gliomas. Bulletin of Siberian Medicine. 2014;13(3):119-125.
Bliseeva АV. Cancer in Russia in 2009 (morbidity and mortality). Мoscow. 2010;17.
Mufazalov FF, Abbasova RR, Mufazalova NA, Goncharova OV. Modern tactics of treatment of malignant glyom of the brain and the case of the complete answer of the tumor on the background of the long-term reception of bevacizumab. Malignant tumours. 2017;(2):33-39.
Sklyar S, Sitovskaya D, Mirolyubova Y, Kushnirova V. Lymphopenia in Patients with Glioblastoma: Literature Review and Case Presentations. Journal of Clinical Surgery and Surgical Research, BioRes Scientia Publishers. 2024;3(3):1-8.
McGirt MJ, Mukherjee D, Chaichana KL, Than KD, Weingart JD, Quinones-Hinojosa A. Association of surgically acquired motor and language deficits on overall survival after resection of glioblastoma multiforme. Neurosurgery. 2009 Sep;65(3):463-9; discussion 469-70. doi: 10.1227/01.NEU.0000349763.42238.E9. PMID: 19687690.
Rynda AY, Olyushin VE, Rostovtsev DM, Zabrodskaya YM, Ulitin AY, Papayan GV. Intraoperative photodynamic therapy in complex treatment of malignant gliomas. Zh Vopr Neirokhir Im N N Burdenko. 2023;87(1):25-34. English, Russian. doi: 10.17116/neiro20238701125. PMID: 36763550.
Ferrés A, Di Somma A, Mosteiro A, Topczewski TE, Roldán P, Pedrosa L, Diao D, Pineda E, Sierra À, Enseñat J, González-Sánchez JJ. Photodynamic therapy in glioblastoma: Detection of intraoperative inadvertent 5-ALA mediated photodynamic therapeutical effect after gross total resection. Front Oncol. 2022 Dec 2;12:1080685. doi: 10.3389/fonc.2022.1080685. PMID: 36531012; PMCID: PMC9755173.
Stupp R, Hegi ME, Mason WP, van den Bent MJ. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459-66.
Strong MJ, Koduri S, Allison JA, Pesavento CM, Ogunsola S, Ogunsola O, Yee TJ, Khalsa SSS, Saadeh YS, Joseph JR, Kashlan ON, Park P, Oppenlander ME, Szerlip NJ. Bone metastasis from glioblastoma: a systematic review. J Neurooncol. 2022 Jul;158(3):379-392. doi: 10.1007/s11060-022-04025-4. Epub 2022 May 17. PMID: 35578056.
Kim AV, Khachatryan VA, Samochernykh KA. Ekstranevral’noe metastazirovanie glioblastomy. Vestnik khirurgii imeni I. I. Grekova. 2007;166 (6), 70–74.
Awan M, Liu S, Sahgal A, Das S, Chao ST, Chang EL, Knisely JP, Redmond K, Sohn JW, Machtay M, Sloan AE, Mansur DB, Rogers LR, Lo SS. Extra-CNS metastasis from glioblastoma: a rare clinical entity. Expert Rev Anticancer Ther. 2015 May;15(5):545-52. doi: 10.1586/14737140.2015.1028374. PMID: 25907706.
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, Soffietti R, von Deimling A, Ellison DW. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol. 2021 Aug 2;23(8):1231-1251. doi: 10.1093/neuonc/noab106. PMID: 34185076; PMCID: PMC8328013.Trashkov AP, Spirin AL, Tsygan NV, Artyomenko MR. Cerebral Glial Tumors: General Principles of Diagnostics and Treatment // Pediatrician (St. Petersburg). 2015;6(4):75-84.
Watanabe T, Vital A, Nobusawa S, Kleihues P, Ohgaki H. Selective acquisition of IDH1 R132C mutations in astrocytomas associated with Li-Fraumeni syndrome. Acta Neuropathol. 2009 Jun;117(6):653-6. doi: 10.1007/s00401-009-0528-x. Epub 2009 Apr 2. PMID: 19340432.
Jin G, Reitman ZJ, Spasojevic I, Batinic-Haberle I, Yang J, Schmidt-Kittler O, Bigner DD, Yan H. 2-hydroxyglutarate production, but not dominant negative function, is conferred by glioma-derived NADP-dependent isocitrate dehydrogenase mutations. PLoS One. 2011 Feb 4;6(2):e16812. doi: 10.1371/journal.pone.0016812. PMID: 21326614; PMCID: PMC3033901.
Garber K. Oncometabolite? IDH1 discoveries raise possibility of new metabolism targets in brain cancers and leukemia. J Natl Cancer Inst. 2010 Jul 7;102(13):926-8. doi: 10.1093/jnci/djq262. Epub 2010 Jun 24. PMID: 20576929.
Nayak L, Reardon DA. High-grade Gliomas. Continuum (Minneap Minn). 2017 Dec;23(6, Neuro-oncology):1548-1563. doi: 10.1212/CON.0000000000000554. PMID: 29200110.
Wilke CM, Wu K, Zhao E, Wang G, Zou W. Prognostic significance of regulatory T cells in tumor. Int J Cancer. 2010 Aug 15;127(4):748-58. doi: 10.1002/ijc.25464. PMID: 20473951.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011 Mar 4;144(5):646-74. doi: 10.1016/j.cell.2011.02.013. PMID: 21376230.
Al-Zoughbi W, Al-Zhoughbi W, Huang J, Paramasivan GS, Till H, Pichler M, Guertl-Lackner B, Hoefler G. Tumor macroenvironment and metabolism. Semin Oncol. 2014 Apr;41(2):281-95. doi: 10.1053/j.seminoncol.2014.02.005. Epub 2014 Mar 1. Erratum in: Semin Oncol. 2014 Aug;41(4):e31. doi: 10.1053/j.seminoncol.2014.07.005. PMID: 24787299; PMCID: PMC4012137.
Sklyar SS, Sitovskaya DA, Mirolyubova IuV, Kushnirova VS. Immune system dysfunction in patients with glioblastoma. Literature review. Clinical cases. Rossiiskii neirokhirurgicheskii zhurnal imeni professora A. L. Polenova. 2023;15(4):200-208.
Zhang W, Blank A, Kremenetskaia I, Nitzsche A. CD13 expression affects glioma patient survival and influences key functions of human glioblastoma cell lines in vitro. BMC Cancer. 2024;22;24(1):369.
Mao P, Joshi K, Li J, Kim SH, Li P, Santana-Santos L, Luthra S, Chandran UR, Benos PV, Smith L, Wang M, Hu B, Cheng SY, Sobol RW, Nakano I. Mesenchymal glioma stem cells are maintained by activated glycolytic metabolism involving aldehyde dehydrogenase 1A3. Proc Natl Acad Sci U S A. 2013 May 21;110(21):8644-9. doi: 10.1073/pnas.1221478110. Epub 2013 May 6. PMID: 23650391; PMCID: PMC3666732.
Bhat KPL, Balasubramaniyan V, Vaillant B, Ezhilarasan R, Hummelink K, Hollingsworth F, Wani K, Heathcock L, James JD, Goodman LD, Conroy S, Long L, Lelic N, Wang S, Gumin J, Raj D, Kodama Y, Raghunathan A, Olar A, Joshi K, Pelloski CE, Heimberger A, Kim SH, Cahill DP, Rao G, Den Dunnen WFA, Boddeke HWGM, Phillips HS, Nakano I, Lang FF, Colman H, Sulman EP, Aldape K. Mesenchymal differentiation mediated by NF-κB promotes radiation resistance in glioblastoma. Cancer Cell. 2013 Sep 9;24(3):331-46. doi: 10.1016/j.ccr.2013.08.001. Epub 2013 Aug 29. PMID: 23993863; PMCID: PMC3817560.
Hu B, Ruan Y, Wei F, Qin G, Mo X, Wang X, Zou D. Identification of three glioblastoma subtypes and a six-gene prognostic risk index based on the expression of growth factors and cytokines. Am J Transl Res. 2020 Aug 15;12(8):4669-4682. PMID: 32913540; PMCID: PMC7476164.
Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, Misra A, Nigro JM, Colman H, Soroceanu L, Williams PM, Modrusan Z, Feuerstein BG, Aldape K. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006 Mar;9(3):157-73. doi: 10.1016/j.ccr.2006.02.019. PMID: 16530701.
Song Y, Zhang Y, Wang X, Han X, Shi M, Xu L, Yu J, Zhang L, Han S. SPI1 activates TGF-β1/PI3K/Akt signaling through transcriptional upregulation of FKBP12 to support the mesenchymal phenotype of glioma stem cells. Brain Pathol. 2024 May;34(3):e13217. doi: 10.1111/bpa.13217. Epub 2023 Oct 22. PMID: 37865975; PMCID: PMC11007049.
Prager BC, Bhargava S, Mahadev V, Hubert CG, Rich JN. Glioblastoma Stem Cells: Driving Resilience through Chaos. Trends Cancer. 2020 Mar;6(3):223-235. doi: 10.1016/j.trecan.2020.01.009. Epub 2020 Feb 3. PMID: 32101725; PMCID: PMC8779821.
Hambardzumyan D, Bergers G. Glioblastoma: Defining Tumor Niches. Trends Cancer. 2015 Dec;1(4):252-265. doi: 10.1016/j.trecan.2015.10.009. PMID: 27088132; PMCID: PMC4831073.
Mohiuddin E, Wakimoto H. Extracellular matrix in glioblastoma: opportunities for emerging therapeutic approaches. Am J Cancer Res. 2021 Aug 15;11(8):3742-3754. PMID: 34522446; PMCID: PMC8414390.
Giles B, Nakhjavani M, Wiesa A, Knight T. Unravelling the Glioblastoma Tumour Microenvironment: Can Aptamer Targeted Delivery Become Successful in Treating Brain Cancers? Cancers (Basel). 2023;1;15(17):4376.
Zhang Y, Zeng A, Liu S, Li R, Wang X, Yan W, Li H, You Y. Genome-wide identification of epithelial-mesenchymal transition-associated microRNAs reveals novel targets for glioblastoma therapy. Oncol Lett. 2018 May;15(5):7625-7630. doi: 10.3892/ol.2018.8280. Epub 2018 Mar 15. PMID: 29740486; PMCID: PMC5934713.
Iwadate Y. Epithelial-mesenchymal transition in glioblastoma progression. Oncol Lett. 2016 Mar;11(3):1615-1620. doi: 10.3892/ol.2016.4113. Epub 2016 Jan 14. PMID: 26998052; PMCID: PMC4774466.
Jäkel S, Dimou L. Glial Cells and Their Function in the Adult Brain: A Journey through the History of Their Ablation. Front Cell Neurosci. 2017 Feb 13;11:24. doi: 10.3389/fncel.2017.00024. PMID: 28243193; PMCID: PMC5303749.
Iser IC, Lenz G, Wink MR. EMT-like process in glioblastomas and reactive astrocytes. Neurochem Int. 2019 Jan;122:139-143. doi: 10.1016/j.neuint.2018.11.016. Epub 2018 Nov 26. PMID: 30496766.
Zhang H, Zhou Y, Cui B, Liu Z, Shen H. Novel insights into astrocyte-mediated signaling of proliferation, invasion and tumor immune microenvironment in glioblastoma. Biomed Pharmacother. 2020 Jun;126:110086. doi: 10.1016/j.biopha.2020.110086. Epub 2020 Mar 12. PMID: 32172060.
Shi L, Wang Z, Rong J, Fei X, Li X, He B, Gong W, Qian J. Inhibition of TGF-β1-induced epithelial-mesenchymal transition in gliomas by DMC-HA. Aging (Albany NY). 2023 Dec 27;15(24):15183-15195. doi: 10.18632/aging.205340. Epub 2023 Dec 27. PMID: 38154100; PMCID: PMC10781457.
Xu X, Hou Y, Long N, Jiang L, Yan Z, Xu Y, Lv Y, Xiang X, Yang H, Liu J, Qi X, Chu L. TPPP3 promote epithelial-mesenchymal transition via Snail1 in glioblastoma. Sci Rep. 2023 Oct 20;13(1):17960. doi: 10.1038/s41598-023-45233-w. PMID: 37863960; PMCID: PMC10589222.
Hambardzumyan D, Gutmann DH, Kettenmann H. The role of microglia and macrophages in glioma maintenance and progression. Nat Neurosci. 2016;19(1):20-7.
Coniglio SJ, Eugenin E, Dobrenis K, Stanley ER, West BL, Symons MH, Segall JE. Microglial stimulation of glioblastoma invasion involves epidermal growth factor receptor (EGFR) and colony stimulating factor 1 receptor (CSF-1R) signaling. Mol Med. 2012 May 9;18(1):519-27. doi: 10.2119/molmed.2011.00217. PMID: 22294205; PMCID: PMC3356419.
Zhang J, Sarkar S, Cua R, Zhou Y, Hader W, Yong VW. A dialog between glioma and microglia that promotes tumor invasiveness through the CCL2/CCR2/interleukin-6 axis. Carcinogenesis. 2012 Feb;33(2):312-9. doi: 10.1093/carcin/bgr289. Epub 2011 Dec 8. PMID: 22159219.
Wick W, Platten M, Weller M. Glioma cell invasion: regulation of metalloproteinase activity by TGF-beta. J Neurooncol. 2001 Jun;53(2):177-85. doi: 10.1023/a:1012209518843. PMID: 11716069.
He X, Guo Y, Yu C, Zhang H, Wang S. Epithelial-mesenchymal transition is the main way in which glioma-associated microglia/macrophages promote glioma progression. Front Immunol. 2023 Mar 10;14:1097880. doi: 10.3389/fimmu.2023.1097880. PMID: 36969175; PMCID: PMC10036378.