
Mastocytosis: Principles and Pitfalls in the Diagnosis of a Unique Disease
Hematology PathologyInternal Medicine受け取った 17 Jul 2024 受け入れられた 05 Aug 2024 オンラインで公開された 06 Aug 2024
Focusing on Biology, Medicine and Engineering ISSN: 2995-8067 | Quick Google Scholar
Next Full Text
Study of the Histological Features of the Stroma of High-Grade Gliomas Depending on the Status of the Mutation in the IDH1 Gene


受け取った 17 Jul 2024 受け入れられた 05 Aug 2024 オンラインで公開された 06 Aug 2024
Mastocytosis, a hematological neoplasm, manifests with diverse clinical, molecular, and histomorphological features. This review explores the different subtypes of mastocytosis, focusing on the role of molecular pathology and histomorphology in diagnosing systemic mastocytosis (SM). Systemic mastocytosis is characterized by histologically confirmed extracutaneous involvement, presenting a diagnostic challenge due to its rarity and diverse subtypes, ranging from occult SM to mast cell leukemia. The complexity of accurate SM diagnosis underscores the necessity for a comprehensive understanding of the disease spectrum. Mastocytosis emerges as a rare, multifaceted disease, predominantly affecting children in the cutaneous form and adults in the systemic variant. The review advocates a multidisciplinary diagnostic approach involving experienced hematopathologists and haematooncologists, employing conventional histomorphology, immunohistochemistry, and molecular techniques. This holistic approach is crucial for accurate diagnosis, especially in light of recent therapeutic advances, particularly the growing importance of tyrosine kinase inhibitors (TKI) in the management of mastocytosis.
Mastocytosis comprises a diverse group of disorders caused by the clonal proliferation of abnormal mast cells, leading to their accumulation in the skin and/or various extracutaneous organs. As a recognition of its distinct biology, mastocytosis was removed from the subtypes of Myeloproliferative Neoplasms (MPN) and is listed as a separate entity in the 5th Edition of the WHO classification [] and in the International Consensus Classification 2022 []. Mastocytosis exhibits a wide range of clinical and histomorphological features and it is essential to distinguish between the pure cutaneous and systemic variants of mastocytosis [-]. Cutaneous Mastocytosis (CM) is limited to the skin, occurs most commonly in children, and generally has a good prognosis [,]. On the other hand, Systemic Mastocytosis (SM) is the most common form of mastocytosis diagnosed in adults and is defined by mast cell infiltration of one or more extracutaneous organs (with or without skin involvement) with a variable prognosis. SM is classified into non-advanced variants (bone marrow mastocytosis or BMM, indolent systemic mastocytosis or ISM, and smoldering systemic mastocytosis or SSM) and advanced variants (aggressive systemic mastocytosis or ASM, systemic mastocytosis with associated haematological neoplasm or SM-AHN, and mast cell leukaemia or MCL). Treatment approaches may focus on managing symptoms or eliminating neoplastic mast cells, with multikinase inhibitors targeting KIT D816V and other key signalling molecules emerging as new options for advanced SM.
Due to the rarity of the disease and the heterogeneity of SM, which often presents with non-specific and overlapping features (ranging from occult SM to mast cell leukemia), establishing a definitive and accurate diagnosis can be very challenging. Comprehensive care for patients with mastocytosis requires a multidisciplinary team approach, and referral to centers with expertise in mastocytosis is strongly recommended.
Table 1.
Diagnostic approach
Using the updated WHO and ICC criteria of 2022 is essential for diagnosing mastocytosis (Table 2).
The histopathological hallmark is a compact infiltrate consisting of a cohesive cluster of at least 15 mast cells (the only major diagnostic criterion).
Additionally, four minor criteria have been defined:
Diagnosis of SM is established when the major criterion and at least 1 minor criterion or at least 3 minor criteria are present.
In over 90% of patients, the diagnosis of Systemic Mastocytosis (SM) relies on histomorphological analysis of bone marrow, pure cytological methods can only be applied in mast cell leukemia []. Basic dyes like Giemsa and Toluidine Blue, along with immunohistochemical stains, are used in this process []. Basic dyes reveal the presence of metachromatic intracytoplasmic granules, which are unique to mast cells and contain a wide range of bioactive mediators. No other human cell contains such metachromatic granules (Figure 1). The metachromatic granules of basophilic granulocytes are water soluble and therefore not detectable in routinely processed tissue using formalin as a fixative and are therefore of no diagnostic relevance in hematopathology.
As atypical mast cells are often hypogranular (or even agranular), it is essential to always use an appropriate antibody panel to confirm or exclude the diagnosis of SM:
The mature immunophenotype of mast cells is defined by the coexpression of tryptase and CD117, a feature consistently present in both normal and reactive (hyperplastic) states []. On the other hand, the expression of aberrant antigens such as CD25 (and, to a lesser extent, CD30) is almost exclusively observed in clonally mutated mast cells within the setting of SM [,] (Figure 1). The only exception to this rule is the occurrence of CD25-expressing (atypical) mast cells in rare cases of myeloid/lymphoid neoplasms with eosinophilia carrying a PDGFR-alpha fusion gene []. It’s important to note that a cell lacking CD117 expression cannot be classified as a mast cell.
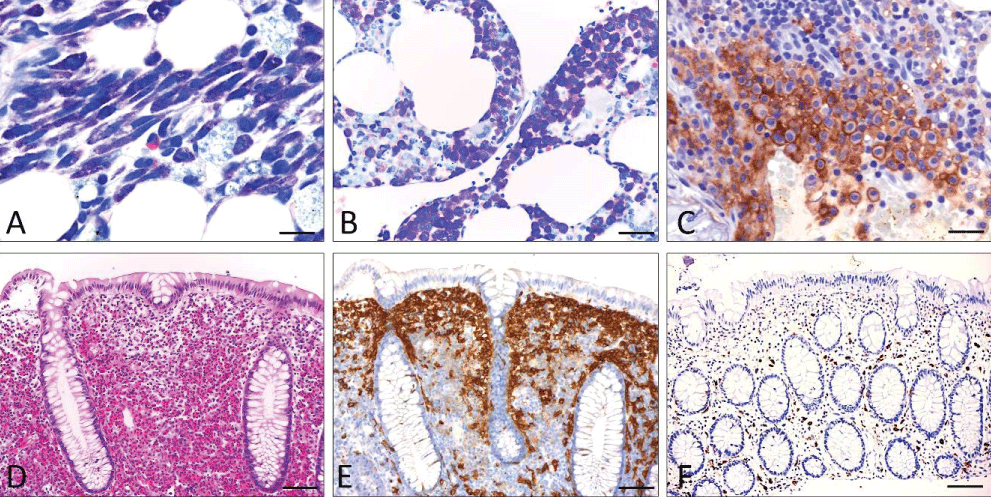 Figure 1: Basic features of SM (A-C; bone marrow infiltrate and D-F; infiltrate of the colon). A) Systemic mastocytosis (undifferentiated subtype). (Giemsa stain): Compact infiltrate consisting of > 15 mast cells exhibiting both spindle shape and moderate hypogranulation. B) Systemic mastocytosis (well-differentiated subtype) (Giemsa stain): Compact infiltrate consisting of >15 mast cells showing both exclusive roundness of the cytoplasm and signs of hypergranulation. C) Anti-CD25 immunohistochemistry: Compact mast cell infiltrates in systemic mastocytosis involving the bone marrow. Strong aberrant expression of CD25 by atypical mast cells. Note the specific annular, membrane-associated immunostaining. D-F) Systemic (intestinal) mastocytosis mimicking eosinophilic colitis with mast cells exhibiting an incomplete-aberrant immunophenotype. D) H&E stain: Lamina propria mucosae is filled with densely packed mature eosinophilic granulocytes leading to an initial diagnosis of „eosinophilic colitis“. E) Anti-CD117 immunohistochemistry: Immunostaining reveals CD117-expressing mast cells forming compact band-like infiltrates in a subepithelial position. F) Anti-tryptase immunohistochemistry: The number of tryptase-expressing mast cells is low obviously reflecting those mast that do not belong to the neoplastic clone. Scale bar A-C 5 m; D-F 50 m.
Figure 1: Basic features of SM (A-C; bone marrow infiltrate and D-F; infiltrate of the colon). A) Systemic mastocytosis (undifferentiated subtype). (Giemsa stain): Compact infiltrate consisting of > 15 mast cells exhibiting both spindle shape and moderate hypogranulation. B) Systemic mastocytosis (well-differentiated subtype) (Giemsa stain): Compact infiltrate consisting of >15 mast cells showing both exclusive roundness of the cytoplasm and signs of hypergranulation. C) Anti-CD25 immunohistochemistry: Compact mast cell infiltrates in systemic mastocytosis involving the bone marrow. Strong aberrant expression of CD25 by atypical mast cells. Note the specific annular, membrane-associated immunostaining. D-F) Systemic (intestinal) mastocytosis mimicking eosinophilic colitis with mast cells exhibiting an incomplete-aberrant immunophenotype. D) H&E stain: Lamina propria mucosae is filled with densely packed mature eosinophilic granulocytes leading to an initial diagnosis of „eosinophilic colitis“. E) Anti-CD117 immunohistochemistry: Immunostaining reveals CD117-expressing mast cells forming compact band-like infiltrates in a subepithelial position. F) Anti-tryptase immunohistochemistry: The number of tryptase-expressing mast cells is low obviously reflecting those mast that do not belong to the neoplastic clone. Scale bar A-C 5 m; D-F 50 m.Primary diagnosis of SM in extramedullary tissues is rare, occurring in <10% of patients. Here, the GI tract mucosa plays a major role [-]. It is important to know that in at least 50% of cases of SM involving the GI tract mucosa (Figure 1), tryptase expression appears to be reduced or even absent, leading to an incomplete aberrant immunophenotype, especially when aberrant expression of CD25 (and/or CD30) is seen. It is therefore highly recommended to use anti-CD117 for initial screening of mast cell numbers in the GI tract mucosa. A primary diagnosis of SM in lymph nodes, liver, or spleen is possible but seems to be a very rare event [,]. Diagnostic criteria for SM should be applied to extramedullary tissues, but precise definitions have not yet been published.
It is crucial to delineate the type of infiltration pattern in order to subtype SM. Conventional stainings such as Giemsa allow a rough orientation but adequate immunohistochemical stainings are a prerequisite for a correct diagnosis in all patients.
The following main types of infiltration do occur:
The diffuse-interstitial infiltration consists of loosely scattered mast cells, sometimes forming small groups, but compact infiltrates are absent. In typical cases of SM, there is a high proportion of spindle-shaped mast cells, sometimes arranged in a meshwork. SM cases presenting with infiltration patterns 1 and 2 are easily overlooked and may cause problems for the diagnosing pathologist. Multifocal infiltration patterns 3 and 4 are the most common and easy to recognize. The compact infiltrates often contain not only mast cells but also a mixture of lymphocytes and eosinophils in varying numbers. Reticulin fibres are always increased and collagen fibrosis may occur. The degree of fibrosis may vary within a patient’s compact infiltrates. Packed is defined by diffuse infiltration with almost complete destruction of the pre-existing microarchitecture.
While the majority of SM patients with diffuse interstitial and multifocal infiltration have completely intact haematopoiesis, in a minority of cases another (non-mast cell) haematological neoplasm can be detected. This is usually a myeloid, rarely a lymphoid neoplasm. Almost all defined subtypes of myeloid neoplasms can occur, with a marked preponderance of Myelodysplastic/
Myeloproliferative Neoplasms (MDS/MPN), of which Chronic Myelomonocytic Leukaemia (CMML) is the most common [].
Please refer to Figure 2 for a diagnostic workup of a patient with suspected mastocytosis.
According to the updated classification system of mastocytosis, the following subtypes of SM can be distinguished (note that cutaneous mastocytosis is strictly limited to the skin and almost always occurs in children):
ISM is the most common subtype of SM (80% of cases) with skin and bone marrow involvement. The skin lesions are macroscopically and histopathologically indistinguishable from cutaneous mastocytosis (urticaria pigmentosa adultorum). ISM is usually associated with infiltration patterns 3 and 4, but pattern 2 may also be seen (20% - 30% of cases). BMM is much rarer than ISM, but the absence of the leading clinical symptom of skin efflorescences and the low degree of infiltration are the reasons why it is often not detected. BMM is usually associated with unexpected osteoporosis in younger and middle-aged males, but also with anaphylactic reactions due to hymenoptera stings showing a strong association with infiltration pattern 2. SSM is a rare subtype with marked multifocal bone marrow infiltration (usually 20% - 30%, or even more), morphologically indistinguishable from ASM, but with at least one clinical B-finding according to WHO definitions.
ASM, MCL, and SM-AHN belong to the so-called group of advanced SM. Among aggressive SM SM-AHN is by far the most common subtype, but is often difficult to diagnose because both disease compartments need to be recognized and correctly defined according to WHO and ICC criteria. All infiltration patterns mentioned above can be seen in SM-AHN. In the rare event of MCL and packed marrow infiltration by atypical mast cells, normal blood cell precursors but also the AHN compartment may be overlooked. The presence of activating point mutations associated with myeloid neoplasms is crucial for the detection of SM-AHN. However, the AHN usually obscures the SM, which may be morphologically almost undetectable in some of these patients (“occult” SM). Typically, SM progresses in the “empty” marrow and thus is recognizable only after cytoreductive therapy. This is especially true for patients with initially diagnosed “KIT D816V-positive acute myeloid leukemia/AML”, which often actually represents SM-AHN/AML. ASM is rare and exhibits multifocal and marked bone marrow infiltration (> 30% - 40%). ASM is a clinicopathological diagnosis because it requires the presence of at least one C-finding. C findings are defined as clinical signs of organ insufficiency due to proven mast cell infiltration. MCL is the only subtype of SM that can be diagnosed on the basis of cytomorphological findings alone. If the number of mast cells in bone marrow smears exceeds 20% of all nucleated cells, or if mast cells represent > 10% of circulating leukocytes in the blood, a diagnosis of MCL can be made at first glance. “Pure” MCL almost always shows diffuse and packed bone marrow infiltration. Leukemic and aleukemic variants of MCL occur based on the number of circulating atypical mast cells (> 10% in leukemic MCL, which is actually very rare). A fascinating disease is the coexistence of MCL and AML (Figure 3 A-E). Due to modern treatment strategies, patients with SM survive much longer than in the past, and the transformation of ISM, SSM, or ASM into a secondary malignancy is rare.
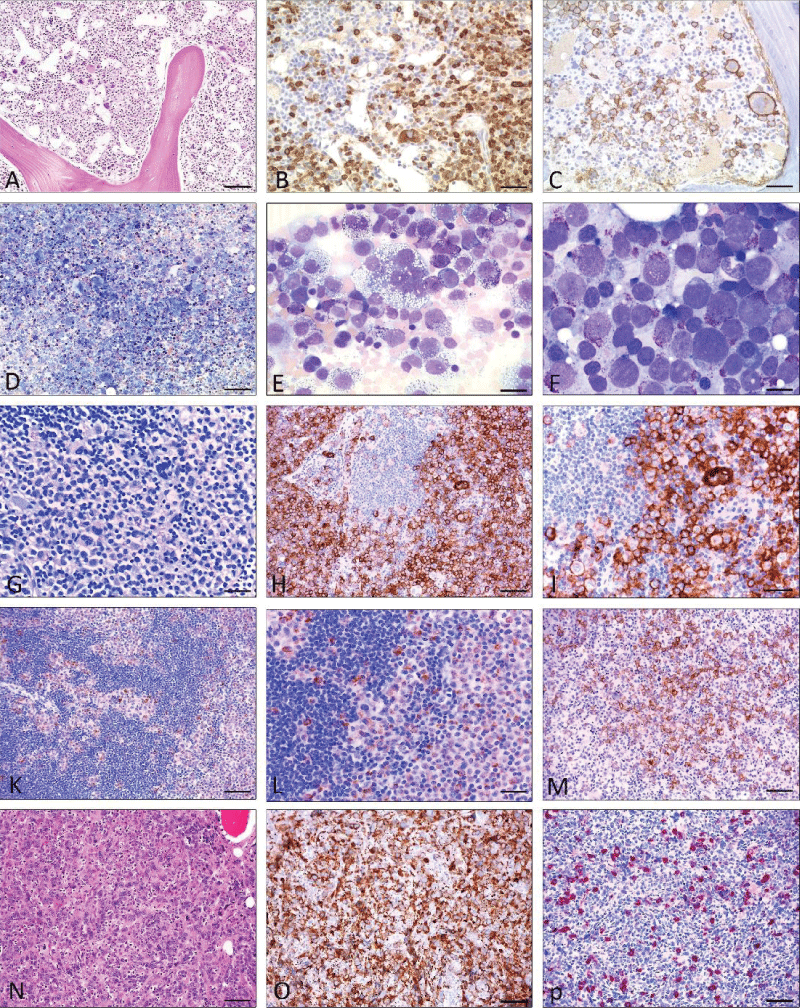 Figure 3: Mast cell leukemia with coexisting AML (A-E), Mast cell sarcoma (G-M), and differential diagnosis myelomastocytic leukemia (F) (19) and (N-P) chronic basophilic leukemia. A) H&E stain: Markedly hypercellular bone marrow diffusely infiltrated by medium-sized blastoid cells. Some loosely scattered megakaryocytes are also shown. Dilated sinuses. B) Anti-tryptase immunohistochemistry: Immunostaining reveals abundant tryptase-positive cells. C) Anti-CD30 immunohistochemistry: Many tumor cells including multinucleated large cells co-express CD30. D -E) Pappenheim stain: Bone marrow smear reveals marked hypercellularity. Higher magnification (E) shows atypical mast cells with vacuolated cytoplasm and metachromatic granules including multinucleated forms accounting for more than 20% of nucleated cells. F: Myelomastocytic leukemia (Pappenheim stain) The bone marrow smear shows an abundance of blast cells with large pale nuclei and few intracytoplamatic metachromatic granules (= metachromatic blast cells). G-M; Mast cell sarcoma: G (Giemsa stain) Tissue from a large intraabdominal mass lesion with clinical suspicion of malignant lymphoma. Conventional staining at first glance is consistent with such a diagnosis, however, only CD117 (H; I) was expressed by tumor cells, and all lymphocytic markers were negative. Additional immunohistochemistry with anti-tryptase (K, L) finally revealed the mast cell nature of the tumor. M) Anti-CD30 immunohistochemistry: A significant proportion of the tumour cells expressed CD30, which could easily have led to a false diagnosis of lymphoma. N-P; Chronic basophilic leukemia in blast crisis (CBL-BC) (N); H&E staining: Bone marrow is extremely hypercellular with focal infiltrates of blastoid cells, strongly expressing CD61 (O). In other parts of the bone marrow, mature granulocytes expressing tryptase are detected (P). Scale bar A-D and G-P 50 m; E-F 5 m.
Figure 3: Mast cell leukemia with coexisting AML (A-E), Mast cell sarcoma (G-M), and differential diagnosis myelomastocytic leukemia (F) (19) and (N-P) chronic basophilic leukemia. A) H&E stain: Markedly hypercellular bone marrow diffusely infiltrated by medium-sized blastoid cells. Some loosely scattered megakaryocytes are also shown. Dilated sinuses. B) Anti-tryptase immunohistochemistry: Immunostaining reveals abundant tryptase-positive cells. C) Anti-CD30 immunohistochemistry: Many tumor cells including multinucleated large cells co-express CD30. D -E) Pappenheim stain: Bone marrow smear reveals marked hypercellularity. Higher magnification (E) shows atypical mast cells with vacuolated cytoplasm and metachromatic granules including multinucleated forms accounting for more than 20% of nucleated cells. F: Myelomastocytic leukemia (Pappenheim stain) The bone marrow smear shows an abundance of blast cells with large pale nuclei and few intracytoplamatic metachromatic granules (= metachromatic blast cells). G-M; Mast cell sarcoma: G (Giemsa stain) Tissue from a large intraabdominal mass lesion with clinical suspicion of malignant lymphoma. Conventional staining at first glance is consistent with such a diagnosis, however, only CD117 (H; I) was expressed by tumor cells, and all lymphocytic markers were negative. Additional immunohistochemistry with anti-tryptase (K, L) finally revealed the mast cell nature of the tumor. M) Anti-CD30 immunohistochemistry: A significant proportion of the tumour cells expressed CD30, which could easily have led to a false diagnosis of lymphoma. N-P; Chronic basophilic leukemia in blast crisis (CBL-BC) (N); H&E staining: Bone marrow is extremely hypercellular with focal infiltrates of blastoid cells, strongly expressing CD61 (O). In other parts of the bone marrow, mature granulocytes expressing tryptase are detected (P). Scale bar A-D and G-P 50 m; E-F 5 m.Mast Cell Sarcoma (MCS) is an extremely rare subvariant of mastocytosis [,]. Two main variants are separated:
Primary MCS has been observed in different tissue sites and apparently is usually misdiagnosed due to its sarcomatous infiltration (Figure 3).
To prove or exclude infiltration of SM in the GI tract mucosa poses a common challenge in patients with Mast Cell Activation Syndrome (MCAS). MCAS, being exceptionally rare, is not diagnosed based on morphology. The main clinical feature is a transient increase of mast cell-mediated mediators in the blood during an allergic or even anaphylactic event, but these increases are of very limited duration []. 90% of mucosal biopsies from the GI tract do not display any signs of SM, both morphologically and molecularly, as KIT p.D816V is typically absent. The count of intramucosal mast cells exhibits significant variability both within individuals and across different individuals. The only proof of SM involving the GI tract mucosa is the presence of compact often band-like, sometimes micronodular mast cell infiltrates in the lamina propria mucosae. In rare instances, mast cells can completely fill the connective tissue of the mucosal layer. Moreover, in at least 50% of patients mast cells exhibit an incomplete-aberrant immunophenotype, lacking tryptase expression (Figure 3). Therefore, anti-tryptase antibodies are not suitable for screening mast numbers in SM patients. The detection of KIT p.D816V completes the diagnosis of SM in the GI tract.
As mentioned in the beginning, the diagnosis of systemic mastocytosis is based on a combination of clinical, histopathological, and molecular criteria. A minor criterion is the demonstration of clonality by the detection of activating mutations of the KIT gene. The KIT p.D816V mutation (located in the Tyrosine Kinase (TK) domain) is found in the majority (90%) of cases of systemic mastocytosis and is considered a hallmark of the disease.
The frequency of KIT mutation in systemic mastocytosis varies according to the subtype. In Indolent Systemic Mastocytosis (ISM), the mutation is detected in more than 90% of cases. In aggressive forms, such as Aggressive Systemic Mastocytosis (ASM) and Mast Cell Leukaemia (MCL), and in WDSM, the mutation is less common [-]. In these subtypes, other KIT mutations outside the TK domain may be detected.
In SM-AHN, the KIT mutation may be present not only in the mast cells but also in the haematopoietic compartment, suggesting a common progenitor []. Usually, SM with multilineage KIT mutation (and high Variant Allele Frequency (VAF)) has an aggressive course, especially if other secondary mutations such as ASXL1, SRSF2, or RUNX1 are present [-]. Particularly in ISM with low VAF, it is important to use sensitive methods such as digital-droplet-PCR [] or suppression-PCR [] for the detection of KIT mutations to avoid false negative results [].
In instances of Indolent Systemic Mastocytosis (ISM), there may be disseminated follicle-like lymphocytic aggregates in close proximity to or intermingled with the neoplastic mast cells. This pattern can give the appearance of a low-grade malignant non-Hodgkin’s lymphoma. At first glance, lymphocytic lymphomas such as Chronic Lymphocytic Leukemia (CLL) and Lymphoplasmacytic Lymphoma (LPL) can be easily misdiagnosed due to their high mast cell content []. The presence of an immature immunophenotype in mast cells, characterized by the co-expression of CD25 and/or CD30, helps to confirm the diagnosis of ISM. On the other hand, an aberrant immunophenotype in the lymphoid compartment, featuring the co-expression of CD20, CD23, and CD5, aids in establishing the diagnosis of CLL. To further support these diagnoses, additional molecular studies can be performed to demonstrate the clonality of mast cells (via KIT p.D816V) or lymphoid cells (through IgH rearrangement or MYD88 p. L265P mutation in LPL).
In a significant number of cases of high-grade systemic mastocytosis (including aggressive systemic mastocytosis, systemic mastocytosis with associated hematological neoplasm, and mast cell leukemia), a majority of neoplastic mast cells express high levels of CD30. Without anti-tryptase staining, there’s a risk of misinterpreting compact mast cell infiltrates as a malignant lymphoma (such as Hodgkin’s sarcoma or anaplastic large cell lymphoma) or even a malignant germ cell tumor [].
Differentiating Systemic Mastocytosis with Associated Hematological Neoplasm and eosinophilia (SM-AHN) from Myeloid/Lymphoid Neoplasms with eosinophilia (MLN-TK) is a challenging task []. This difficulty arises because atypical mast cell infiltrates with the expression of CD25 may be prominent in myeloid/lymphoid neoplasms carrying a PDGFR-alpha fusion gene. Additional molecular analysis is essential; detection of either the PDGFR-alpha fusion gene (MLN-TK) or the KIT p.D816V mutation (SM-AHN) is critical for correct diagnosis.
The main differential diagnosis of MCL is myelomastocytic leukemia [] (MML, Figure 2). MML represents an advanced myeloid neoplasm characterized by an increased presence of blasts, as well as a significant rise in immature mast cells and/or metachromatic blast cells (comprising more than 10% of nucleated cells in bone marrow smears). Mast cells in MML typically express CD117 and tryptase but lack CD2 and CD25. While the KIT mutant p. D816V is not detectable, other KIT mutations are sporadically identified. In contrast to the spindle-shaped mast cells and compact focal infiltrates commonly found in Systemic Mastocytosis (SM), mast cells in MML are neither spindle-shaped nor do they form compact focal infiltrates, presenting a clear contrast. Immunohistochemical markers recommended for suspected MML include CD34, CD117, tryptase, and CD25.
Additionally, distinguishing basophilic leukemia from MCL can pose a challenge due to the abundance of round tryptase-expressing neoplastic cells in both conditions (Figure 3). However, a key differentiation lies in the expression of CD117 (KIT) – a marker present on mast cells of MCL but absent on neoplastic basophils in basophilic leukemia.
Mastocytosis is a rare multifaceted disease with a broad clinical and histomorphological spectrum including various subtypes, in particular (pure) cutaneous (almost always children) and systemic variants (usually adults). Therefore, a multidisciplinary approach with experienced hematopathologists and haemato-oncologists using conventional histomorphology, immunohistochemistry, and molecular techniques is required for correct diagnosis. A correct diagnosis is crucial for the patient regarding recent developments in therapy with special emphasis on Tyrosine Kinase Inhibitors (TKI).
Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, Bejar R, Berti E, Busque L, Chan JKC, Chen W, Chen X, Chng WJ, Choi JK, Colmenero I, Coupland SE, Cross NCP, De Jong D, Elghetany MT, Takahashi E, Emile JF, Ferry J, Fogelstrand L, Fontenay M, Germing U, Gujral S, Haferlach T, Harrison C, Hodge JC, Hu S, Jansen JH, Kanagal-Shamanna R, Kantarjian HM, Kratz CP, Li XQ, Lim MS, Loeb K, Loghavi S, Marcogliese A, Meshinchi S, Michaels P, Naresh KN, Natkunam Y, Nejati R, Ott G, Padron E, Patel KP, Patkar N, Picarsic J, Platzbecker U, Roberts I, Schuh A, Sewell W, Siebert R, Tembhare P, Tyner J, Verstovsek S, Wang W, Wood B, Xiao W, Yeung C, Hochhaus A. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia. 2022 Jul;36(7):1703-1719. doi: 10.1038/s41375-022-01613-1. Epub 2022 Jun 22. PMID: 35732831; PMCID: PMC9252913.
Arber DA, Orazi A, Hasserjian RP, Borowitz MJ, Calvo KR, Kvasnicka HM, Wang SA, Bagg A, Barbui T, Branford S, Bueso-Ramos CE, Cortes JE, Dal Cin P, DiNardo CD, Dombret H, Duncavage EJ, Ebert BL, Estey EH, Facchetti F, Foucar K, Gangat N, Gianelli U, Godley LA, Gökbuget N, Gotlib J, Hellström-Lindberg E, Hobbs GS, Hoffman R, Jabbour EJ, Kiladjian JJ, Larson RA, Le Beau MM, Loh ML, Löwenberg B, Macintyre E, Malcovati L, Mullighan CG, Niemeyer C, Odenike OM, Ogawa S, Orfao A, Papaemmanuil E, Passamonti F, Porkka K, Pui CH, Radich JP, Reiter A, Rozman M, Rudelius M, Savona MR, Schiffer CA, Schmitt-Graeff A, Shimamura A, Sierra J, Stock WA, Stone RM, Tallman MS, Thiele J, Tien HF, Tzankov A, Vannucchi AM, Vyas P, Wei AH, Weinberg OK, Wierzbowska A, Cazzola M, Döhner H, Tefferi A. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: integrating morphologic, clinical, and genomic data. Blood. 2022 Sep 15;140(11):1200-1228. doi: 10.1182/blood.2022015850. PMID: 35767897; PMCID: PMC9479031.
Pieri L, Bonadonna P, Elena C, Papayannidis C, Grifoni FI, Rondoni M, Girlanda S, Mauro M, Magliacane D, Elli EM, Iorno ML, Almerigogna F, Scarfì F, Salerno R, Fanelli T, Gesullo F, Corbizi Fattori G, Bonifacio M, Perbellini O, Artuso A, Soverini S, De Benedittis C, Muratori S, Pravettoni V, Cova V, Cortellini G, Ciceri F, Cortelezzi A, Martinelli G, Triggiani M, Merante S, Vannucchi AM, Zanotti R. Clinical presentation and management practice of systemic mastocytosis. A survey on 460 Italian patients. Am J Hematol. 2016 Jul;91(7):692-9. doi: 10.1002/ajh.24382. Epub 2016 Jun 2. PMID: 27060898.
Valent P, Akin C, Hartmann K, Alvarez-Twose I, Brockow K, Hermine O, Niedoszytko M, Schwaab J, Lyons JJ, Carter MC, Elberink HO, Butterfield JH, George TI, Greiner G, Ustun C, Bonadonna P, Sotlar K, Nilsson G, Jawhar M, Siebenhaar F, Broesby-Olsen S, Yavuz S, Zanotti R, Lange M, Nedoszytko B, Hoermann G, Castells M, Radia DH, Muñoz-Gonzalez JI, Sperr WR, Triggiani M, Kluin-Nelemans HC, Galli SJ, Schwartz LB, Reiter A, Orfao A, Gotlib J, Arock M, Horny HP, Metcalfe DD. Updated Diagnostic Criteria and Classification of Mast Cell Disorders: A Consensus Proposal. Hemasphere. 2021 Oct 13;5(11):e646. doi: 10.1097/HS9.0000000000000646. PMID: 34901755; PMCID: PMC8659997.
Fuchs D, Kilbertus A, Kofler K, von Bubnoff N, Shoumariyeh K, Zanotti R, Bonadonna P, Scaffidi L, Doubek M, Elberink HO, Span LFR, Hermine O, Elena C, Benvenuti P, Yavuz AS, Brockow K, Zink A, Aberer E, Gorska A, Romantowski J, Hadzijusufovic E, Fortina AB, Caroppo F, Perkins C, Illerhaus A, Panse J, Vucinic V, Jawhar M, Sabato V, Triggiani M, Parente R, Bergström A, Breynaert C, Gotlib J, Reiter A, Hartmann K, Niedoszytko M, Arock M, Kluin-Nelemans HC, Sperr WR, Greul R, Valent P. Scoring the Risk of Having Systemic Mastocytosis in Adult Patients with Mastocytosis in the Skin. J Allergy Clin Immunol Pract. 2021 Apr;9(4):1705-1712.e4. doi: 10.1016/j.jaip.2020.12.022. Epub 2020 Dec 23. PMID: 33346151.
Wolff K, Komar M, Petzelbauer P. Clinical and histopathological aspects of cutaneous mastocytosis. Leuk Res. 2001 Jul;25(7):519-28. doi: 10.1016/s0145-2126(01)00044-3. PMID: 11377676.
Hartmann K, Henz BM. Cutaneous mastocytosis -- clinical heterogeneity. Int Arch Allergy Immunol. 2002 Feb;127(2):143-6. doi: 10.1159/000048187. PMID: 11919426.
Sperr WR, Escribano L, Jordan JH, Schernthaner GH, Kundi M, Horny HP, Valent P. Morphologic properties of neoplastic mast cells: delineation of stages of maturation and implication for cytological grading of mastocytosis. Leuk Res. 2001 Jul;25(7):529-36. doi: 10.1016/s0145-2126(01)00041-8. PMID: 11377677.
Horny HP, Valent P. Diagnosis of mastocytosis: general histopathological aspects, morphological criteria, and immunohistochemical findings. Leuk Res. 2001 Jul;25(7):543-51. doi: 10.1016/s0145-2126(01)00021-2. PMID: 11377679.
Horny HP, Sillaber C, Menke D, Kaiserling E, Wehrmann M, Stehberger B, Chott A, Lechner K, Lennert K, Valent P. Diagnostic value of immunostaining for tryptase in patients with mastocytosis. Am J Surg Pathol. 1998 Sep;22(9):1132-40. doi: 10.1097/00000478-199809000-00013. PMID: 9737247.
Escribano L, Orfao A, Díaz-Agustin B, Villarrubia J, Cerveró C, López A, Marcos MA, Bellas C, Fernández-Cañadas S, Cuevas M, Sánchez A, Velasco JL, Navarro JL, Miguel JF. Indolent systemic mast cell disease in adults: immunophenotypic characterization of bone marrow mast cells and its diagnostic implications. Blood. 1998 Apr 15;91(8):2731-6. PMID: 9531582.
Morgado JM, Perbellini O, Johnson RC, Teodósio C, Matito A, Álvarez-Twose I, Bonadonna P, Zamò A, Jara-Acevedo M, Mayado A, Garcia-Montero A, Mollejo M, George TI, Zanotti R, Orfao A, Escribano L, Sánchez-Muñoz L. CD30 expression by bone marrow mast cells from different diagnostic variants of systemic mastocytosis. Histopathology. 2013 Dec;63(6):780-7. doi: 10.1111/his.12221. Epub 2013 Sep 20. PMID: 24111625.
Pardanani A, Brockman SR, Paternoster SF, Flynn HC, Ketterling RP, Lasho TL, Ho CL, Li CY, Dewald GW, Tefferi A. FIP1L1-PDGFRA fusion: prevalence and clinicopathologic correlates in 89 consecutive patients with moderate to severe eosinophilia. Blood. 2004 Nov 15;104(10):3038-45. doi: 10.1182/blood-2004-03-0787. Epub 2004 Jul 29. PMID: 15284118.
Lübke J, Naumann N, Hoffmann O, Horny HP, Sotlar K, Rudelius M, Metzgeroth G, Fabarius A, Hofmann WK, Reiter A, Schwaab J. A clinical, morphological and molecular study of 70 patients with gastrointestinal involvement in systemic mastocytosis. Sci Rep. 2024 Jan 6;14(1):702. doi: 10.1038/s41598-023-49749-z. PMID: 38184670; PMCID: PMC10771518.
Genta RM, Turner KO, Collins MH, Wechsler JB, Arva NC, Pletneva MA, Dellon ES, Walker MM. Quantification of Mucosal Mast Cells in the Gastrointestinal Tract: A Primer for Practicing Pathologists. Arch Pathol Lab Med. 2024 Feb 1;148(2):e25-e35. doi: 10.5858/arpa.2023-0070-OA. PMID: 37450346.
Panarelli NC, Hornick JL, Yantiss RK. What Is the Value of Counting Mast Cells in Gastrointestinal Mucosal Biopsies? Mod Pathol. 2023 Feb;36(2):100005. doi: 10.1016/j.modpat.2022.100005. Epub 2023 Jan 9. PMID: 36853780.
Doyle LA, Hornick JL. Pathology of extramedullary mastocytosis. Immunol Allergy Clin North Am. 2014 May;34(2):323-39. doi: 10.1016/j.iac.2014.01.010. PMID: 24745677.
Horny HP, Ruck MT, Kaiserling E. Spleen findings in generalized mastocytosis. A clinicopathologic study. Cancer. 1992 Jul 15;70(2):459-68. doi: 10.1002/1097-0142(19920715)70:2<459::aid-cncr2820700214>3.0.co;2-4. PMID: 1617595.
Valent P, Sperr WR, Samorapoompichit P, Geissler K, Lechner K, Horny HP, Bennett JM. Myelomastocytic overlap syndromes: biology, criteria, and relationship to mastocytosis. Leuk Res. 2001 Jul;25(7):595-602. doi: 10.1016/s0145-2126(01)00040-6. PMID: 11377685.
Gülen T, Sander B, Nilsson G, Palmblad J, Sotlar K, Horny HP, Hägglund H. Systemic mastocytosis: progressive evolution of an occult disease into fatal mast cell leukemia: unique findings on an unusual hematological neoplasm. Med Oncol. 2012 Dec;29(5):3540-6. doi: 10.1007/s12032-012-0261-5. Epub 2012 Jun 3. PMID: 22661384.
Krauth MT, Födinger M, Rebuzzi L, Greul R, Chott A, Valent P. Aggressive systemic mastocytosis with sarcoma-like growth in the skeleton, leukemic progression, and partial loss of mast cell differentiation antigens. Haematologica. 2007 Dec;92(12):e126-9. doi: 10.3324/haematol.11996. PMID: 18055976.
Valent P, Hartmann K, Bonadonna P, Gülen T, Brockow K, et al. Global Classification of Mast Cell Activation Disorders: An ICD-10-CM-Adjusted Proposal of the ECNM-AIM Consortium. J Allergy Clin Immunol Pract. 2022 Aug;10(8):1941-1950. doi: 10.1016/j.jaip.2022.05.007. Epub 2022 May 25. PMID: 35623575.
Garcia-Montero AC, Jara-Acevedo M, Teodosio C, Sanchez ML, Nunez R, Prados A, Aldanondo I, Sanchez L, Dominguez M, Botana LM, Sanchez-Jimenez F, Sotlar K, Almeida J, Escribano L, Orfao A. KIT mutation in mast cells and other bone marrow hematopoietic cell lineages in systemic mast cell disorders: a prospective study of the Spanish Network on Mastocytosis (REMA) in a series of 113 patients. Blood. 2006 Oct 1;108(7):2366-72. doi: 10.1182/blood-2006-04-015545. Epub 2006 Jun 1. PMID: 16741248.
Arock M, Sotlar K, Akin C, Broesby-Olsen S, Hoermann G, Escribano L, Kristensen TK, Kluin-Nelemans HC, Hermine O, Dubreuil P, Sperr WR, Hartmann K, Gotlib J, Cross NC, Haferlach T, Garcia-Montero A, Orfao A, Schwaab J, Triggiani M, Horny HP, Metcalfe DD, Reiter A, Valent P. KIT mutation analysis in mast cell neoplasms: recommendations of the European Competence Network on Mastocytosis. Leukemia. 2015 Jun;29(6):1223-32. doi: 10.1038/leu.2015.24. Epub 2015 Feb 4. PMID: 25650093; PMCID: PMC4522520.
Álvarez-Twose I, Jara-Acevedo M, Morgado JM, García-Montero A, Sánchez-Muñoz L, Teodósio C, Matito A, Mayado A, Caldas C, Mollejo M, Orfao A, Escribano L. Clinical, immunophenotypic, and molecular characteristics of well-differentiated systemic mastocytosis. J Allergy Clin Immunol. 2016 Jan;137(1):168-178.e1. doi: 10.1016/j.jaci.2015.05.008. Epub 2015 Jun 19. PMID: 26100086.
Escribano L, Alvarez-Twose I, Sánchez-Muñoz L, Garcia-Montero A, Núñez R, Almeida J, Jara-Acevedo M, Teodósio C, García-Cosío M, Bellas C, Orfao A. Prognosis in adult indolent systemic mastocytosis: a long-term study of the Spanish Network on Mastocytosis in a series of 145 patients. J Allergy Clin Immunol. 2009 Sep;124(3):514-21. doi: 10.1016/j.jaci.2009.05.003. Epub 2009 Jul 9. PMID: 19541349.
Schwaab J, Schnittger S, Sotlar K, Walz C, Fabarius A, Pfirrmann M, Kohlmann A, Grossmann V, Meggendorfer M, Horny HP, Valent P, Jawhar M, Teichmann M, Metzgeroth G, Erben P, Ernst T, Hochhaus A, Haferlach T, Hofmann WK, Cross NC, Reiter A. Comprehensive mutational profiling in advanced systemic mastocytosis. Blood. 2013 Oct 3;122(14):2460-6. doi: 10.1182/blood-2013-04-496448. Epub 2013 Aug 19. PMID: 23958953.
Jawhar M, Schwaab J, Schnittger S, Meggendorfer M, Pfirrmann M, Sotlar K, Horny HP, Metzgeroth G, Kluger S, Naumann N, Haferlach C, Haferlach T, Valent P, Hofmann WK, Fabarius A, Cross NC, Reiter A. Additional mutations in SRSF2, ASXL1 and/or RUNX1 identify a high-risk group of patients with KIT D816V(+) advanced systemic mastocytosis. Leukemia. 2016 Jan;30(1):136-43. doi: 10.1038/leu.2015.284. Epub 2015 Oct 14. PMID: 26464169.
Muñoz-González JI, Jara-Acevedo M, Alvarez-Twose I, Merker JD, Teodosio C, Hou Y, Henriques A, Roskin KM, Sanchez-Muñoz L, Tsai AG, Caldas C, Matito A, Sánchez-Gallego JI, Mayado A, Dasilva-Freire N, Gotlib JR, Escribano L, Orfao A, García-Montero AC. Impact of somatic and germline mutations on the outcome of systemic mastocytosis. Blood Adv. 2018 Nov 13;2(21):2814-2828. doi: 10.1182/bloodadvances.2018020628. PMID: 30373888; PMCID: PMC6234367.
Greiner G, Gurbisz M, Ratzinger F, Witzeneder N, Simonitsch-Klupp I, Mitterbauer-Hohendanner G, Mayerhofer M, Müllauer L, Sperr WR, Valent P, Hoermann G. Digital PCR: A Sensitive and Precise Method for KITD816V Quantification in Mastocytosis. Clin Chem. 2018 Mar;64(3):547-555. doi: 10.1373/clinchem.2017.277897. Epub 2017 Dec 13. PMID: 29237714; PMCID: PMC7115889.
Sotlar K, Escribano L, Landt O, Möhrle S, Herrero S, Torrelo A, Lass U, Horny HP, Bültmann B. One-step detection of c-kit point mutations using peptide nucleic acid-mediated polymerase chain reaction clamping and hybridization probes. Am J Pathol. 2003 Mar;162(3):737-46. doi: 10.1016/S0002-9440(10)63870-9. PMID: 12598308; PMCID: PMC1868096.
Hoermann G, Sotlar K, Jawhar M, Kristensen T, Bachelot G, Nedoszytko B, et al. Standards of Genetic Testing in the Diagnosis and Prognostication of Systemic Mastocytosis in 2022: Recommendations of the EU-US Cooperative Group. J Allergy Clin Immunol Pract. 2022 Aug;10(8):1953-1963. doi: 10.1016/j.jaip.2022.03.001. Epub 2022 Mar 11. PMID: 35283331.
Horny HP, Sotlar K, Stellmacher F, Valent P, Grabbe J. An unusual case of systemic mastocytosis associated with chronic lymphocytic leukaemia (SM-CLL). J Clin Pathol. 2006 Mar;59(3):264-8. doi: 10.1136/jcp.2005.026989. PMID: 16505276; PMCID: PMC1860346.
Sotlar K, Cerny-Reiterer S, Petat-Dutter K, Hessel H, Berezowska S, Müllauer L, Valent P, Horny HP. Aberrant expression of CD30 in neoplastic mast cells in high-grade mastocytosis. Mod Pathol. 2011 Apr;24(4):585-95. doi: 10.1038/modpathol.2010.224. Epub 2010 Dec 24. PMID: 21186345.
Horny HP, Sotlar K, Reiter A, Valent P. Myelomastocytic leukemia: histopathological features, diagnostic criteria and differential diagnosis. Expert Rev Hematol. 2014 Aug;7(4):431-7. doi: 10.1586/17474086.2014.942280. PMID: 25025369.
Rudelius M, Horny HP. Mastocytosis: Principles and Pitfalls in the Diagnosis of a Unique Disease. IgMin Res. August 06, 2024; 2(8): 694-701. IgMin ID: igmin234; DOI: 10.61927/igmin234; Available at: igmin.link/p234
次のリンクを共有した人は、このコンテンツを読むことができます:
Address Correspondence:
Martina Rudelius, Institute of Pathology, Ludwig Maximilian University of Munich, Munich, Germany, Email: [email protected]
How to cite this article:
Rudelius M, Horny HP. Mastocytosis: Principles and Pitfalls in the Diagnosis of a Unique Disease. IgMin Res. August 06, 2024; 2(8): 694-701. IgMin ID: igmin234; DOI: 10.61927/igmin234; Available at: igmin.link/p234
Copyright: © 2024 Rudelius M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
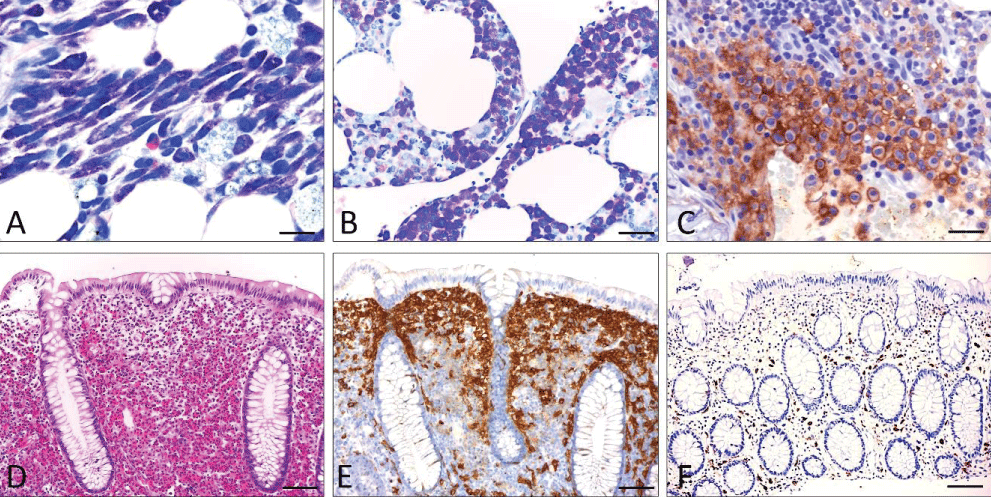 Figure 1: Basic features of SM (A-C; bone marrow infiltrate ...
Figure 1: Basic features of SM (A-C; bone marrow infiltrate ...
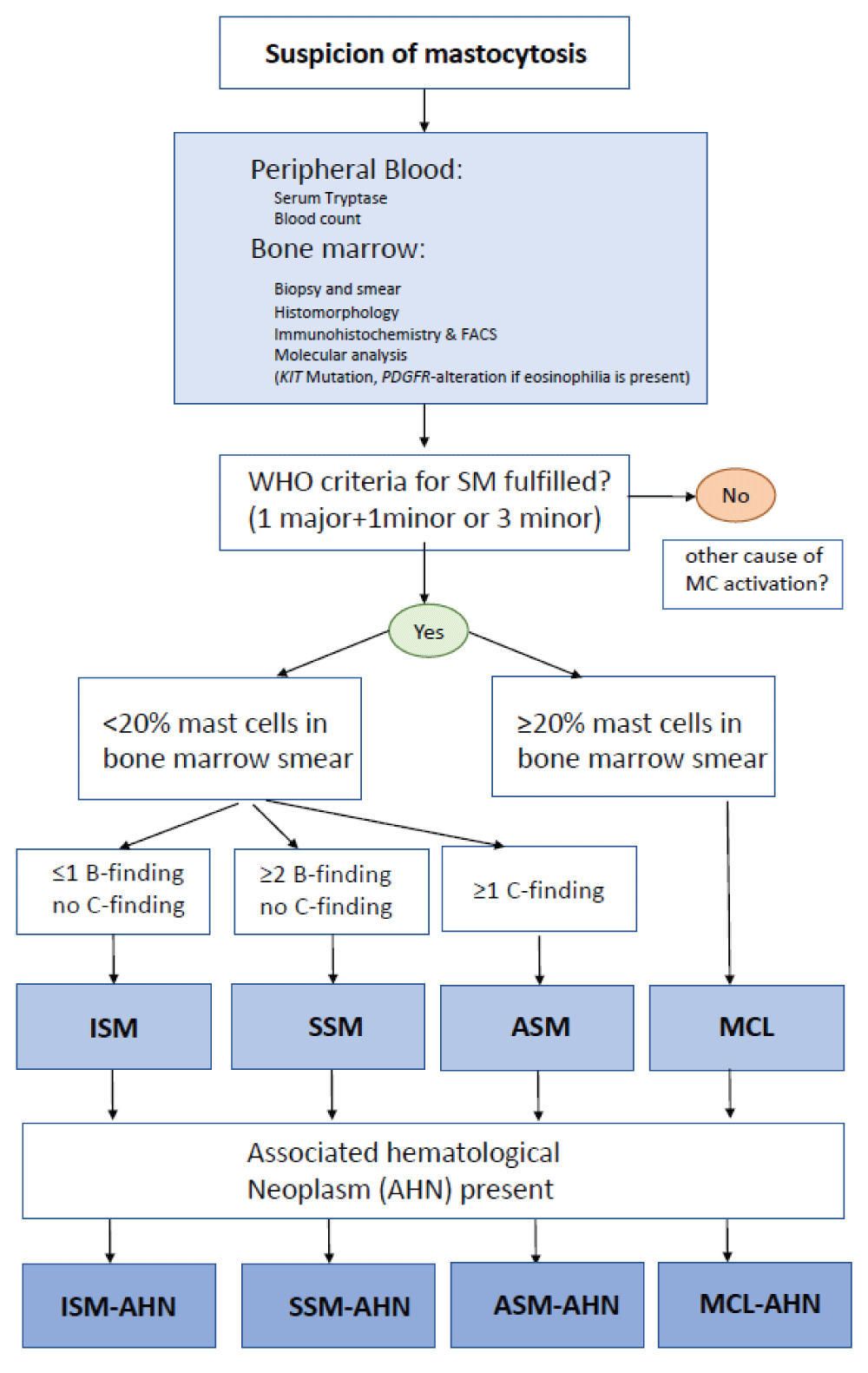 Figure 2: Diagnostic workup of a patient with suspected mast...
Figure 2: Diagnostic workup of a patient with suspected mast...
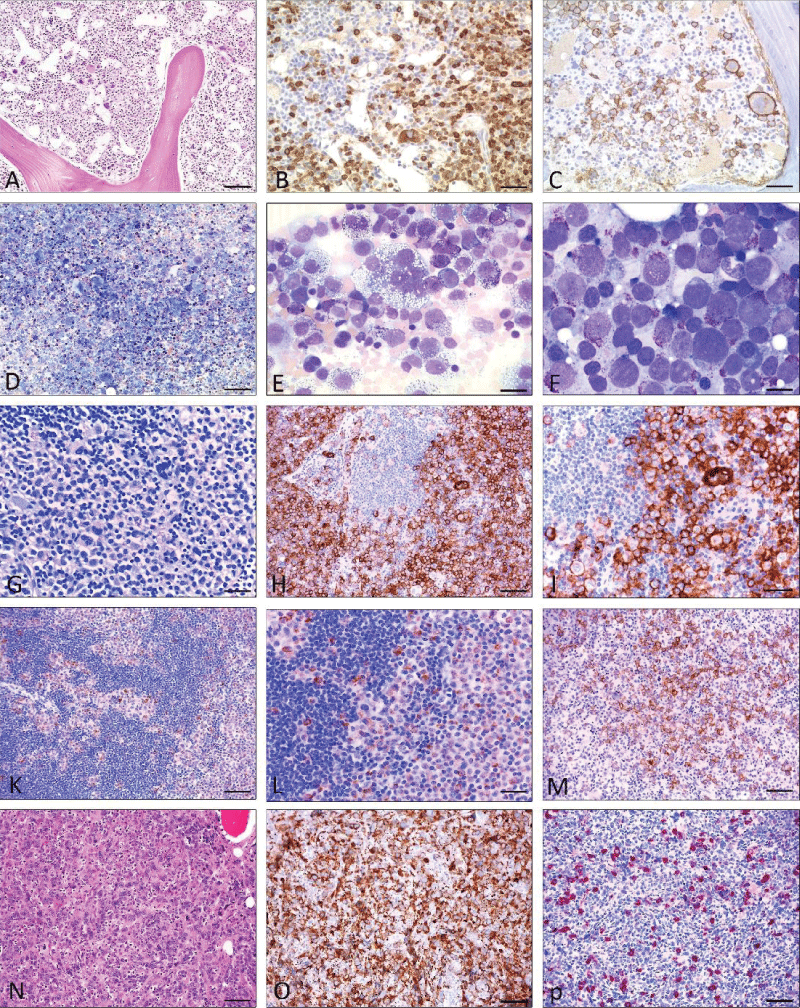 Figure 3: Mast cell leukemia with coexisting AML (A-E), Mast...
Figure 3: Mast cell leukemia with coexisting AML (A-E), Mast...
 Table 1: Classification of mastocytosis....
Table 1: Classification of mastocytosis....
 Table 2: Diagnostic criteria of systemic mastocytosis accor...
Table 2: Diagnostic criteria of systemic mastocytosis accor...
Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, Bejar R, Berti E, Busque L, Chan JKC, Chen W, Chen X, Chng WJ, Choi JK, Colmenero I, Coupland SE, Cross NCP, De Jong D, Elghetany MT, Takahashi E, Emile JF, Ferry J, Fogelstrand L, Fontenay M, Germing U, Gujral S, Haferlach T, Harrison C, Hodge JC, Hu S, Jansen JH, Kanagal-Shamanna R, Kantarjian HM, Kratz CP, Li XQ, Lim MS, Loeb K, Loghavi S, Marcogliese A, Meshinchi S, Michaels P, Naresh KN, Natkunam Y, Nejati R, Ott G, Padron E, Patel KP, Patkar N, Picarsic J, Platzbecker U, Roberts I, Schuh A, Sewell W, Siebert R, Tembhare P, Tyner J, Verstovsek S, Wang W, Wood B, Xiao W, Yeung C, Hochhaus A. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia. 2022 Jul;36(7):1703-1719. doi: 10.1038/s41375-022-01613-1. Epub 2022 Jun 22. PMID: 35732831; PMCID: PMC9252913.
Arber DA, Orazi A, Hasserjian RP, Borowitz MJ, Calvo KR, Kvasnicka HM, Wang SA, Bagg A, Barbui T, Branford S, Bueso-Ramos CE, Cortes JE, Dal Cin P, DiNardo CD, Dombret H, Duncavage EJ, Ebert BL, Estey EH, Facchetti F, Foucar K, Gangat N, Gianelli U, Godley LA, Gökbuget N, Gotlib J, Hellström-Lindberg E, Hobbs GS, Hoffman R, Jabbour EJ, Kiladjian JJ, Larson RA, Le Beau MM, Loh ML, Löwenberg B, Macintyre E, Malcovati L, Mullighan CG, Niemeyer C, Odenike OM, Ogawa S, Orfao A, Papaemmanuil E, Passamonti F, Porkka K, Pui CH, Radich JP, Reiter A, Rozman M, Rudelius M, Savona MR, Schiffer CA, Schmitt-Graeff A, Shimamura A, Sierra J, Stock WA, Stone RM, Tallman MS, Thiele J, Tien HF, Tzankov A, Vannucchi AM, Vyas P, Wei AH, Weinberg OK, Wierzbowska A, Cazzola M, Döhner H, Tefferi A. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: integrating morphologic, clinical, and genomic data. Blood. 2022 Sep 15;140(11):1200-1228. doi: 10.1182/blood.2022015850. PMID: 35767897; PMCID: PMC9479031.
Pieri L, Bonadonna P, Elena C, Papayannidis C, Grifoni FI, Rondoni M, Girlanda S, Mauro M, Magliacane D, Elli EM, Iorno ML, Almerigogna F, Scarfì F, Salerno R, Fanelli T, Gesullo F, Corbizi Fattori G, Bonifacio M, Perbellini O, Artuso A, Soverini S, De Benedittis C, Muratori S, Pravettoni V, Cova V, Cortellini G, Ciceri F, Cortelezzi A, Martinelli G, Triggiani M, Merante S, Vannucchi AM, Zanotti R. Clinical presentation and management practice of systemic mastocytosis. A survey on 460 Italian patients. Am J Hematol. 2016 Jul;91(7):692-9. doi: 10.1002/ajh.24382. Epub 2016 Jun 2. PMID: 27060898.
Valent P, Akin C, Hartmann K, Alvarez-Twose I, Brockow K, Hermine O, Niedoszytko M, Schwaab J, Lyons JJ, Carter MC, Elberink HO, Butterfield JH, George TI, Greiner G, Ustun C, Bonadonna P, Sotlar K, Nilsson G, Jawhar M, Siebenhaar F, Broesby-Olsen S, Yavuz S, Zanotti R, Lange M, Nedoszytko B, Hoermann G, Castells M, Radia DH, Muñoz-Gonzalez JI, Sperr WR, Triggiani M, Kluin-Nelemans HC, Galli SJ, Schwartz LB, Reiter A, Orfao A, Gotlib J, Arock M, Horny HP, Metcalfe DD. Updated Diagnostic Criteria and Classification of Mast Cell Disorders: A Consensus Proposal. Hemasphere. 2021 Oct 13;5(11):e646. doi: 10.1097/HS9.0000000000000646. PMID: 34901755; PMCID: PMC8659997.
Fuchs D, Kilbertus A, Kofler K, von Bubnoff N, Shoumariyeh K, Zanotti R, Bonadonna P, Scaffidi L, Doubek M, Elberink HO, Span LFR, Hermine O, Elena C, Benvenuti P, Yavuz AS, Brockow K, Zink A, Aberer E, Gorska A, Romantowski J, Hadzijusufovic E, Fortina AB, Caroppo F, Perkins C, Illerhaus A, Panse J, Vucinic V, Jawhar M, Sabato V, Triggiani M, Parente R, Bergström A, Breynaert C, Gotlib J, Reiter A, Hartmann K, Niedoszytko M, Arock M, Kluin-Nelemans HC, Sperr WR, Greul R, Valent P. Scoring the Risk of Having Systemic Mastocytosis in Adult Patients with Mastocytosis in the Skin. J Allergy Clin Immunol Pract. 2021 Apr;9(4):1705-1712.e4. doi: 10.1016/j.jaip.2020.12.022. Epub 2020 Dec 23. PMID: 33346151.
Wolff K, Komar M, Petzelbauer P. Clinical and histopathological aspects of cutaneous mastocytosis. Leuk Res. 2001 Jul;25(7):519-28. doi: 10.1016/s0145-2126(01)00044-3. PMID: 11377676.
Hartmann K, Henz BM. Cutaneous mastocytosis -- clinical heterogeneity. Int Arch Allergy Immunol. 2002 Feb;127(2):143-6. doi: 10.1159/000048187. PMID: 11919426.
Sperr WR, Escribano L, Jordan JH, Schernthaner GH, Kundi M, Horny HP, Valent P. Morphologic properties of neoplastic mast cells: delineation of stages of maturation and implication for cytological grading of mastocytosis. Leuk Res. 2001 Jul;25(7):529-36. doi: 10.1016/s0145-2126(01)00041-8. PMID: 11377677.
Horny HP, Valent P. Diagnosis of mastocytosis: general histopathological aspects, morphological criteria, and immunohistochemical findings. Leuk Res. 2001 Jul;25(7):543-51. doi: 10.1016/s0145-2126(01)00021-2. PMID: 11377679.
Horny HP, Sillaber C, Menke D, Kaiserling E, Wehrmann M, Stehberger B, Chott A, Lechner K, Lennert K, Valent P. Diagnostic value of immunostaining for tryptase in patients with mastocytosis. Am J Surg Pathol. 1998 Sep;22(9):1132-40. doi: 10.1097/00000478-199809000-00013. PMID: 9737247.
Escribano L, Orfao A, Díaz-Agustin B, Villarrubia J, Cerveró C, López A, Marcos MA, Bellas C, Fernández-Cañadas S, Cuevas M, Sánchez A, Velasco JL, Navarro JL, Miguel JF. Indolent systemic mast cell disease in adults: immunophenotypic characterization of bone marrow mast cells and its diagnostic implications. Blood. 1998 Apr 15;91(8):2731-6. PMID: 9531582.
Morgado JM, Perbellini O, Johnson RC, Teodósio C, Matito A, Álvarez-Twose I, Bonadonna P, Zamò A, Jara-Acevedo M, Mayado A, Garcia-Montero A, Mollejo M, George TI, Zanotti R, Orfao A, Escribano L, Sánchez-Muñoz L. CD30 expression by bone marrow mast cells from different diagnostic variants of systemic mastocytosis. Histopathology. 2013 Dec;63(6):780-7. doi: 10.1111/his.12221. Epub 2013 Sep 20. PMID: 24111625.
Pardanani A, Brockman SR, Paternoster SF, Flynn HC, Ketterling RP, Lasho TL, Ho CL, Li CY, Dewald GW, Tefferi A. FIP1L1-PDGFRA fusion: prevalence and clinicopathologic correlates in 89 consecutive patients with moderate to severe eosinophilia. Blood. 2004 Nov 15;104(10):3038-45. doi: 10.1182/blood-2004-03-0787. Epub 2004 Jul 29. PMID: 15284118.
Lübke J, Naumann N, Hoffmann O, Horny HP, Sotlar K, Rudelius M, Metzgeroth G, Fabarius A, Hofmann WK, Reiter A, Schwaab J. A clinical, morphological and molecular study of 70 patients with gastrointestinal involvement in systemic mastocytosis. Sci Rep. 2024 Jan 6;14(1):702. doi: 10.1038/s41598-023-49749-z. PMID: 38184670; PMCID: PMC10771518.
Genta RM, Turner KO, Collins MH, Wechsler JB, Arva NC, Pletneva MA, Dellon ES, Walker MM. Quantification of Mucosal Mast Cells in the Gastrointestinal Tract: A Primer for Practicing Pathologists. Arch Pathol Lab Med. 2024 Feb 1;148(2):e25-e35. doi: 10.5858/arpa.2023-0070-OA. PMID: 37450346.
Panarelli NC, Hornick JL, Yantiss RK. What Is the Value of Counting Mast Cells in Gastrointestinal Mucosal Biopsies? Mod Pathol. 2023 Feb;36(2):100005. doi: 10.1016/j.modpat.2022.100005. Epub 2023 Jan 9. PMID: 36853780.
Doyle LA, Hornick JL. Pathology of extramedullary mastocytosis. Immunol Allergy Clin North Am. 2014 May;34(2):323-39. doi: 10.1016/j.iac.2014.01.010. PMID: 24745677.
Horny HP, Ruck MT, Kaiserling E. Spleen findings in generalized mastocytosis. A clinicopathologic study. Cancer. 1992 Jul 15;70(2):459-68. doi: 10.1002/1097-0142(19920715)70:2<459::aid-cncr2820700214>3.0.co;2-4. PMID: 1617595.
Valent P, Sperr WR, Samorapoompichit P, Geissler K, Lechner K, Horny HP, Bennett JM. Myelomastocytic overlap syndromes: biology, criteria, and relationship to mastocytosis. Leuk Res. 2001 Jul;25(7):595-602. doi: 10.1016/s0145-2126(01)00040-6. PMID: 11377685.
Gülen T, Sander B, Nilsson G, Palmblad J, Sotlar K, Horny HP, Hägglund H. Systemic mastocytosis: progressive evolution of an occult disease into fatal mast cell leukemia: unique findings on an unusual hematological neoplasm. Med Oncol. 2012 Dec;29(5):3540-6. doi: 10.1007/s12032-012-0261-5. Epub 2012 Jun 3. PMID: 22661384.
Krauth MT, Födinger M, Rebuzzi L, Greul R, Chott A, Valent P. Aggressive systemic mastocytosis with sarcoma-like growth in the skeleton, leukemic progression, and partial loss of mast cell differentiation antigens. Haematologica. 2007 Dec;92(12):e126-9. doi: 10.3324/haematol.11996. PMID: 18055976.
Valent P, Hartmann K, Bonadonna P, Gülen T, Brockow K, et al. Global Classification of Mast Cell Activation Disorders: An ICD-10-CM-Adjusted Proposal of the ECNM-AIM Consortium. J Allergy Clin Immunol Pract. 2022 Aug;10(8):1941-1950. doi: 10.1016/j.jaip.2022.05.007. Epub 2022 May 25. PMID: 35623575.
Garcia-Montero AC, Jara-Acevedo M, Teodosio C, Sanchez ML, Nunez R, Prados A, Aldanondo I, Sanchez L, Dominguez M, Botana LM, Sanchez-Jimenez F, Sotlar K, Almeida J, Escribano L, Orfao A. KIT mutation in mast cells and other bone marrow hematopoietic cell lineages in systemic mast cell disorders: a prospective study of the Spanish Network on Mastocytosis (REMA) in a series of 113 patients. Blood. 2006 Oct 1;108(7):2366-72. doi: 10.1182/blood-2006-04-015545. Epub 2006 Jun 1. PMID: 16741248.
Arock M, Sotlar K, Akin C, Broesby-Olsen S, Hoermann G, Escribano L, Kristensen TK, Kluin-Nelemans HC, Hermine O, Dubreuil P, Sperr WR, Hartmann K, Gotlib J, Cross NC, Haferlach T, Garcia-Montero A, Orfao A, Schwaab J, Triggiani M, Horny HP, Metcalfe DD, Reiter A, Valent P. KIT mutation analysis in mast cell neoplasms: recommendations of the European Competence Network on Mastocytosis. Leukemia. 2015 Jun;29(6):1223-32. doi: 10.1038/leu.2015.24. Epub 2015 Feb 4. PMID: 25650093; PMCID: PMC4522520.
Álvarez-Twose I, Jara-Acevedo M, Morgado JM, García-Montero A, Sánchez-Muñoz L, Teodósio C, Matito A, Mayado A, Caldas C, Mollejo M, Orfao A, Escribano L. Clinical, immunophenotypic, and molecular characteristics of well-differentiated systemic mastocytosis. J Allergy Clin Immunol. 2016 Jan;137(1):168-178.e1. doi: 10.1016/j.jaci.2015.05.008. Epub 2015 Jun 19. PMID: 26100086.
Escribano L, Alvarez-Twose I, Sánchez-Muñoz L, Garcia-Montero A, Núñez R, Almeida J, Jara-Acevedo M, Teodósio C, García-Cosío M, Bellas C, Orfao A. Prognosis in adult indolent systemic mastocytosis: a long-term study of the Spanish Network on Mastocytosis in a series of 145 patients. J Allergy Clin Immunol. 2009 Sep;124(3):514-21. doi: 10.1016/j.jaci.2009.05.003. Epub 2009 Jul 9. PMID: 19541349.
Schwaab J, Schnittger S, Sotlar K, Walz C, Fabarius A, Pfirrmann M, Kohlmann A, Grossmann V, Meggendorfer M, Horny HP, Valent P, Jawhar M, Teichmann M, Metzgeroth G, Erben P, Ernst T, Hochhaus A, Haferlach T, Hofmann WK, Cross NC, Reiter A. Comprehensive mutational profiling in advanced systemic mastocytosis. Blood. 2013 Oct 3;122(14):2460-6. doi: 10.1182/blood-2013-04-496448. Epub 2013 Aug 19. PMID: 23958953.
Jawhar M, Schwaab J, Schnittger S, Meggendorfer M, Pfirrmann M, Sotlar K, Horny HP, Metzgeroth G, Kluger S, Naumann N, Haferlach C, Haferlach T, Valent P, Hofmann WK, Fabarius A, Cross NC, Reiter A. Additional mutations in SRSF2, ASXL1 and/or RUNX1 identify a high-risk group of patients with KIT D816V(+) advanced systemic mastocytosis. Leukemia. 2016 Jan;30(1):136-43. doi: 10.1038/leu.2015.284. Epub 2015 Oct 14. PMID: 26464169.
Muñoz-González JI, Jara-Acevedo M, Alvarez-Twose I, Merker JD, Teodosio C, Hou Y, Henriques A, Roskin KM, Sanchez-Muñoz L, Tsai AG, Caldas C, Matito A, Sánchez-Gallego JI, Mayado A, Dasilva-Freire N, Gotlib JR, Escribano L, Orfao A, García-Montero AC. Impact of somatic and germline mutations on the outcome of systemic mastocytosis. Blood Adv. 2018 Nov 13;2(21):2814-2828. doi: 10.1182/bloodadvances.2018020628. PMID: 30373888; PMCID: PMC6234367.
Greiner G, Gurbisz M, Ratzinger F, Witzeneder N, Simonitsch-Klupp I, Mitterbauer-Hohendanner G, Mayerhofer M, Müllauer L, Sperr WR, Valent P, Hoermann G. Digital PCR: A Sensitive and Precise Method for KITD816V Quantification in Mastocytosis. Clin Chem. 2018 Mar;64(3):547-555. doi: 10.1373/clinchem.2017.277897. Epub 2017 Dec 13. PMID: 29237714; PMCID: PMC7115889.
Sotlar K, Escribano L, Landt O, Möhrle S, Herrero S, Torrelo A, Lass U, Horny HP, Bültmann B. One-step detection of c-kit point mutations using peptide nucleic acid-mediated polymerase chain reaction clamping and hybridization probes. Am J Pathol. 2003 Mar;162(3):737-46. doi: 10.1016/S0002-9440(10)63870-9. PMID: 12598308; PMCID: PMC1868096.
Hoermann G, Sotlar K, Jawhar M, Kristensen T, Bachelot G, Nedoszytko B, et al. Standards of Genetic Testing in the Diagnosis and Prognostication of Systemic Mastocytosis in 2022: Recommendations of the EU-US Cooperative Group. J Allergy Clin Immunol Pract. 2022 Aug;10(8):1953-1963. doi: 10.1016/j.jaip.2022.03.001. Epub 2022 Mar 11. PMID: 35283331.
Horny HP, Sotlar K, Stellmacher F, Valent P, Grabbe J. An unusual case of systemic mastocytosis associated with chronic lymphocytic leukaemia (SM-CLL). J Clin Pathol. 2006 Mar;59(3):264-8. doi: 10.1136/jcp.2005.026989. PMID: 16505276; PMCID: PMC1860346.
Sotlar K, Cerny-Reiterer S, Petat-Dutter K, Hessel H, Berezowska S, Müllauer L, Valent P, Horny HP. Aberrant expression of CD30 in neoplastic mast cells in high-grade mastocytosis. Mod Pathol. 2011 Apr;24(4):585-95. doi: 10.1038/modpathol.2010.224. Epub 2010 Dec 24. PMID: 21186345.
Horny HP, Sotlar K, Reiter A, Valent P. Myelomastocytic leukemia: histopathological features, diagnostic criteria and differential diagnosis. Expert Rev Hematol. 2014 Aug;7(4):431-7. doi: 10.1586/17474086.2014.942280. PMID: 25025369.