
Levetiracetam-induced Rhabdomyolysis - A Rare Complication
Neurology Clinical Medicine受け取った 07 Jul 2024 受け入れられた 19 Jul 2024 オンラインで公開された 22 Jul 2024
ISSN: 2995-8067 | Quick Google Scholar


受け取った 07 Jul 2024 受け入れられた 19 Jul 2024 オンラインで公開された 22 Jul 2024
Background: Levetiracetam is an anti-epileptic drug that works by modulation of synaptic neurotransmitter release through binding to the synaptic vesicle protein 2A. Levetiracetam is generally well tolerated. Common side effects of levetiracetam include lethargy, drowsiness, headaches, and mood changes. Rhabdomyolysis and an increase in Creatine Kinase (CK) levels are one of the rarely reported effects of levetiracetam.
Case presentation: We present a case of a 20-year-old patient with previously known epilepsy (non-compliant with medicine) who presented with a seizure. The patient was given 1 gram of levetiracetam in the emergency department and was kept at a maintenance dose of 500 mg twice a day. The workup revealed acute kidney injury and raised levels of creatinine kinase that peaked at 19,757 IU/L 72 hours after levetiracetam was started. Levetiracetam was later switched to lamotrigine and aggressive intravenous hydration was done. Gradual improvement of CK level was noted accompanied by improvement in renal function.
Conclusion: Only a few cases of levetiracetam-induced rhabdomyolysis have been reported worldwide. Our case report highlights the importance of monitoring creatinine and CK levels while treating patients with levetiracetam as rhabdomyolysis can be asymptomatic with a rise in CK levels only.
Levetiracetam is a well-known antiepileptic agent that is used to treat generalized or focal-onset seizures. Common side effects include drowsiness, lethargy, headache, and personality changes like irritability and nervousness. Uncommonly, it has been observed to cause elevated Creatine Kinase (CK) levels, and to date, only a few cases of levetiracetam-induced rhabdomyolysis have been reported in the literature [].
Rhabdomyolysis is a fatal condition that occurs secondary to the breakdown of muscle tissue, and the release of the muscle tissue contents like CK, myoglobin, lactate, and aldolase into the blood. CK levels in the blood increase within 2-12 hours of muscle breakdown. An established definition is an increase of serum CK activity of at least 10 times the upper limit of normal []. Patients may be asymptomatic. Some may present with severe electrolyte imbalance, myoglobinuria, acute kidney injury, and disseminated intravascular coagulation. Only 10% of patients present with the classical triad of myoglobinuria, myalgias, and muscle weakness []. It usually occurs in people after exercise, trauma, severe dehydration, long periods of inactivity, heat stroke, neuroleptic malignant syndrome, muscular dystrophies, alcohol, and certain medications like statins []. Rarely, it occurs after the use of anti-epileptic agents like levetiracetam.
Herein, we report a case of a young man in his early 20s who developed significantly elevated CK levels and raised creatinine levels associated with levetiracetam.
We report a case of a young male in his early 20’s, known to have epilepsy since the age of 08 years who presented to the emergency department with an episode of seizure 12 hours ago. The attendant described this episode as turning of the head to the right side followed by stiffening of all four limbs that lasted for 2-3 minutes. It was associated with tongue bite, urinary incontinence, and postictal confusion for 15 minutes. There was even amnesia as well. There was no history of fever, cough, ear discharge, headache, fall, trauma, limb weakness, visual or gait disturbance. The patient had a similar episode of seizure around four years ago, which lasted for 5 minutes and was self-abortive. He was prescribed levetiracetam, but he was non-compliant with it. The last dose that he took was a month ago.
On examination, the patient was of average height and build, blood pressure was 120/80mm Hg, heart rate was 90/min, and he was afebrile. On neurological examination, he was awake, alert, and following commands. The speech was fluent, and cranial nerves were intact. Motor and sensory examinations were also unremarkable. Signs of meningeal irritation were absent.
His baseline workup sent after an hour of the loading dose of levetiracetam showed a deranged creatinine of 2.5 mg/dl. A Magnetic Resonance Imaging (MRI) brain with contrast and Electroencephalogram (EEG) was done that was normal. Due to the deranged creatinine, Nephrology was taken on board, and they advised to get Creatinine Kinase (CK) levels and Aldolase levels done and they were normal. CK level sent 7 hours after the levetiracetam dose was 1219 IU/L (Range: 46-171) and aldolase was 96.7 U/L (Range: 2.5-10). Urine for toxicology was sent to rule out any substance abuse, and it was negative. He also complained of myalgias. The patient was hydrated well, and strict Input-output charting was done. The next day his creatinine level rose to 3.2mg/dl (Figure 1) and on Day 3, his CK peaked at a level of 19757 IU/L (Figure 2).
The patient was loaded with 1 gram of levetiracetam when he first arrived in the emergency department and then was kept on a maintenance dose of 500 mg twice a day. On day 3, when his CK peaked at a level of 19757 IU/L, levetiracetam was switched to lamotrigine. Within 24 hours of stopping levetiracetam, his CK levels and creatinine started to decline. The patient remained stable and was sent home on tablet lamotrigine with the advice to maintain good oral hydration.
He was seen in the clinic regularly after discharge and his symptoms resolved. On his follow-up visit after one month, his CK levels and creatinine were normal.
The case was observed in Pakistan. To the best of our knowledge is the second case observed in Pakistan []. Levetiracetam in recent years has become a widely prescribed anti-epileptic drug to control acute onset seizures considering its broad spectrum, easy availability, low interactions, and limited side effect profile. Thus, it is important to know some of the rarer but critical adverse effects associated with its use
To date, only 15 cases of levetiracetam-induced rhabdomyolysis have been reported worldwide [,]. Akiyama H and colleagues described the first case of levetiracetam-induced rhabdomyolysis in 2014 in a 29-year-old woman with generalized tonic-clonic seizures []. In 2022, a case reported a 34-year-old epileptic female on carbamazepine who presented with status epilepticus. She was given IV levetiracetam 3000 mg, followed by a maintenance dose of 1000 mg BID. However, she developed oliguria, leading to the discontinuation of levetiracetam on day 4. Her CK levels gradually decreased, returning to baseline by day 15 [].
A detailed review of all 15 cases has shown that rhabdomyolysis after the use of levetiracetam was seen in adults ranging from 16 years to 62 years, with most of the patients in their 3rd decade. There was a male preponderance and none of these patients had a prior history of using levetiracetam. The time for CK levels to peak ranged from 1 to 15 days. 33% of the patients had AKI while 40% of the patients had associated myalgias. All these cases showed a down-trending CK level within 24-48 hours of discontinuing levetiracetam except the one reported by Di Lorenzo and Li where the CK levels peaked at 48 hours after discontinuing levetiracetam and then continued to decline [].
Our patient was previously on levetiracetam, but he was non-compliant with the medication. He was given a loading dose of 1 g of levetiracetam and CK level was done 7 hours after the dose. It was 1219 IU/L. The peak CK levels were achieved at 72 hours. He also developed AKI and myalgias. CK declined within 24 hours after discontinuation of levetiracetam. Since significant improvement was noted in the CK level once levetiracetam was switched, muscle biopsy was not considered at that time. As the patient remained seizure-free since his hospital admission and had no known underlying musculoskeletal disorder or any other risk factor that could have contributed to the rhabdomyolysis, we attribute the rhabdomyolysis to be due to levetiracetam.
Levetiracetam works by modulating the synaptic neurotransmitter release through binding to the Synaptic Vesicle Glycoprotein (SV2A) in the brain []. The mechanism by which it causes rhabdomyolysis is still unclear. However, one theory suggests that the interaction of levetiracetam with SV2A protein in the motor nerve terminals of slow muscle fibers causes increased cholinergic neurotransmission. This puts stress on the muscles and increases their breakdown, thereby leading to rhabdomyolysis [].
CK levels are measured in blood. There is, however, no FDA mandate for routine CK monitoring in patients on levetiracetam. While renal dysfunction is a risk factor, our case report highlights that it is important to monitor CK levels in all patients on levetiracetam, especially those who develop muscle pain, weakness, r acute oliguria, even without pre-existing renal issues, due to the risk of asymptomatic rhabdomyolysis. Monitoring CK levels in patients with such conditions shows awareness of levetiracetam’s rare association with rhabdomyolysis. If CK levels are not measured, it suggests this association is not widely recognized.
Apart from levetiracetam, rhabdomyolysis has also been reported with phenytoin, pregabalin, and sodium valproate [-]. Some other drugs can also cause rhabdomyolysis like 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase inhibitors, macrolide antibiotics, and benzodiazepines. Though the exact mechanisms are unclear, mitochondrial dysfunction and calcium homeostasis dysregulation appear to be common factors in drug-induced rhabdomyolysis [].
Brigo, et al. noted that seizures typically cause a mild CPK elevation (< 180 U/L) with peaks at 36-40 hours. In this patient, however, CPK levels were significantly higher and peaked 72 hours after admission [].
Rhabdomyolysis is a rare side effect of levetiracetam and is a life-threatening condition. Our case report highlights the importance of monitoring creatinine and CK levels while treating patients with levetiracetam as rhabdomyolysis can be asymptomatic with a rise in CK levels only. It also emphasizes the fact that patients on levetiracetam presenting with muscle aches/ fatigue should undergo thorough assessment, including testing for high CK levels and monitoring for this complication should be a part of the routine care. Switching to a different anti-epileptic drug should be considered if CK levels rise significantly to avoid kidney injury.
Moinuddin IA. Suspected Levetiracetam-Induced Rhabdomyolysis: A Case Report and Literature Review. Am J Case Rep. 2020 Oct 28;21:e926064. doi: 10.12659/AJCR.926064. PMID: 33112844; PMCID: PMC7603803.
Tiglis M, Hurmuzache T, Bologa C, Neagu TP, Mirea L, Grintescu IM. Rhabdomyolysis-Induced Acute Renal Injury in a Schizophrenic Patient. J Crit Care Med (Targu Mures). 2020 Nov 7;6(4):249-252. doi: 10.2478/jccm-2020-0032. PMID: 33200097; PMCID: PMC7648442.
Bosch X, Poch E, Grau JM. Rhabdomyolysis and acute kidney injury. N Engl J Med. 2009 Jul 2;361(1):62-72. doi: 10.1056/NEJMra0801327. Erratum in: N Engl J Med. 2011 May 19;364(20):1982. PMID: 19571284.
Torres PA, Helmstetter JA, Kaye AM, Kaye AD. Rhabdomyolysis: pathogenesis, diagnosis, and treatment. Ochsner J. 2015 Spring;15(1):58-69. PMID: 25829882; PMCID: PMC4365849.
Shahbaz N, Younus SM, Khan SA, Ain Q-, Khan MA, Memon MH. Levetiracetam Induced Increase in Creatine Phosphokinase Levels. J Coll Physicians Surg Pak. 2017 Mar;27(3):S63-S64. PMID: 28302251.
Akiyama H, Haga Y, Sasaki N, Yanagisawa T, Hasegawa Y. A case of rhabdomyolysis in which levetiracetam was suspected as the cause. Epilepsy Behav Case Rep. 2014 Sep 16;2:152-155. doi: 10.1016/j.ebcr.2014.08.001. PMID: 25667895; PMCID: PMC4308062.
Al-Quliti K, Alhujeily RM. Acute kidney injury with levetiracetam in patient with epilepsy: a case report. Case Reports in Clinical Medicine. 2022;11(3):48-54.
Di Lorenzo R, Li Y. Rhabdomyolysis associated with levetiracetam administration. Muscle Nerve. 2017 Jul;56(1):E1-E2. doi: 10.1002/mus.25548. Epub 2017 Mar 23. PMID: 28039868.
Abou-Khalil B. Levetiracetam in the treatment of epilepsy. Neuropsychiatr Dis Treat. 2008 Jun;4(3):507-523. doi: 10.2147/ndt.s2937. PMID: 18830435; PMCID: PMC2526377.
Carnovale C, Gentili M, Antoniazzi S, Clementi E, Radice S. Levetiracetam-induced rhabdomyolysis: Analysis of reports from the Food and Drug Administration's Adverse Event Reporting System database. Muscle Nerve. 2017 Dec;56(6):E176-E178. doi: 10.1002/mus.25972. Epub 2017 Sep 30. PMID: 28888059.
Kim H, Jo S, Park KW, Han SH, Lee SA. A Case of Phenytoin-induced Rhabdomyolysis in Status Epilepticus. J Epilepsy Res. 2016 Jun 30;6(1):36-38. doi: 10.14581/jer.16007. PMID: 27390679; PMCID: PMC4933680.
Prendergast BD, George CF. Drug-induced rhabdomyolysis--mechanisms and management. Postgrad Med J. 1993 May;69(811):333-336. doi: 10.1136/pgmj.69.811.333. PMID: 8393995; PMCID: PMC2399808.
Gunathilake R, Boyce LE, Knight AT. Pregabalin-associated rhabdomyolysis. Med J Aust. 2013;199(9):624-625.
Kazmi JS, Albarghouthy N, Ramsaywak R. Levetiracetam-Induced Rhabdomyolysis Reversed by Discontinuation: A Case Report. Cureus. 2023;15(11):e48955. doi: 10.7759/cureus.48955. PMID: 38111426; PMCID: PMC10726084.
Nizamudeen K, Sabu ST, Dharan SS. An overview of the rare and life-threatening adverse effects of levetiracetam. World Journal of Pharmaceutical Research. 9:649-670. DOI: 10.20959/wjpr202011-18761
Khan AF, Shaikh N, Abdy F, Kanwar D. Levetiracetam-induced Rhabdomyolysis - A Rare Complication. IgMin Res. July 22, 2024; 2(7): 642-645. IgMin ID: igmin228; DOI: 10.61927/igmin228; Available at: igmin.link/p228
次のリンクを共有した人は、このコンテンツを読むことができます:
Address Correspondence:
Ayisha Farooq Khan, Section of Neurology, Department of Medicine, Aga Khan University, National Stadium Road, Karachi, Pakistan, Email: [email protected]
How to cite this article:
Khan AF, Shaikh N, Abdy F, Kanwar D. Levetiracetam-induced Rhabdomyolysis - A Rare Complication. IgMin Res. July 22, 2024; 2(7): 642-645.
IgMin ID: igmin228; DOI: 10.61927/igmin228; Available at: igmin.link/p228
Copyright: © 2024 Khan AF, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
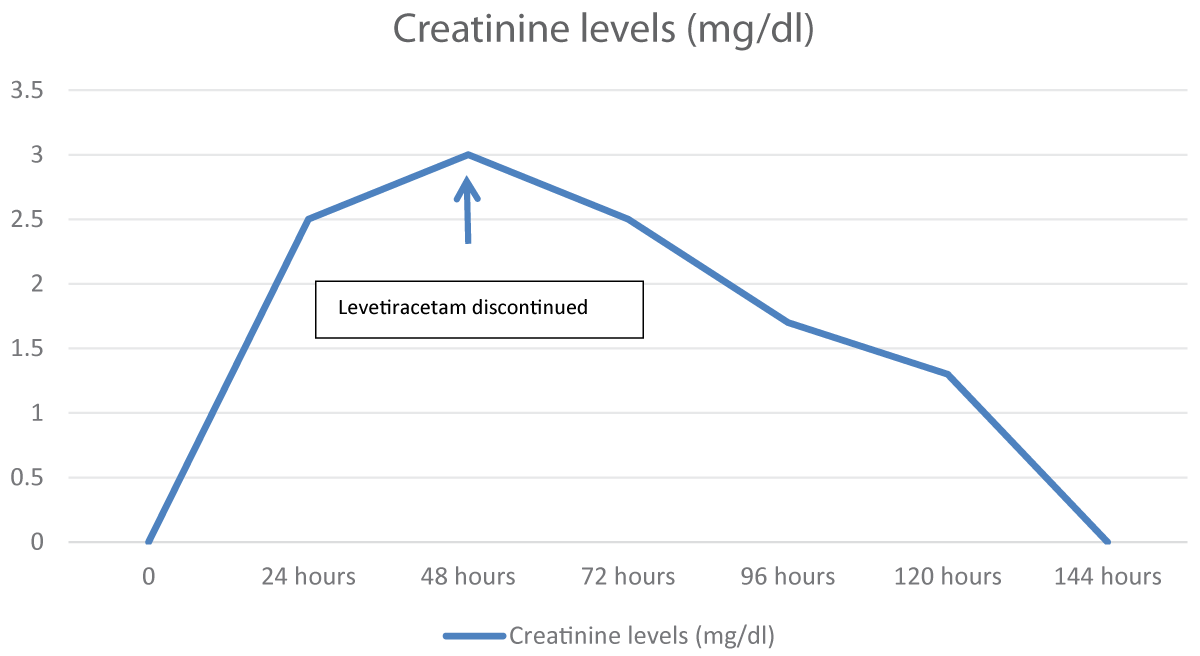 Figure 1: Creatinine level trend in association with levetir...
Figure 1: Creatinine level trend in association with levetir...
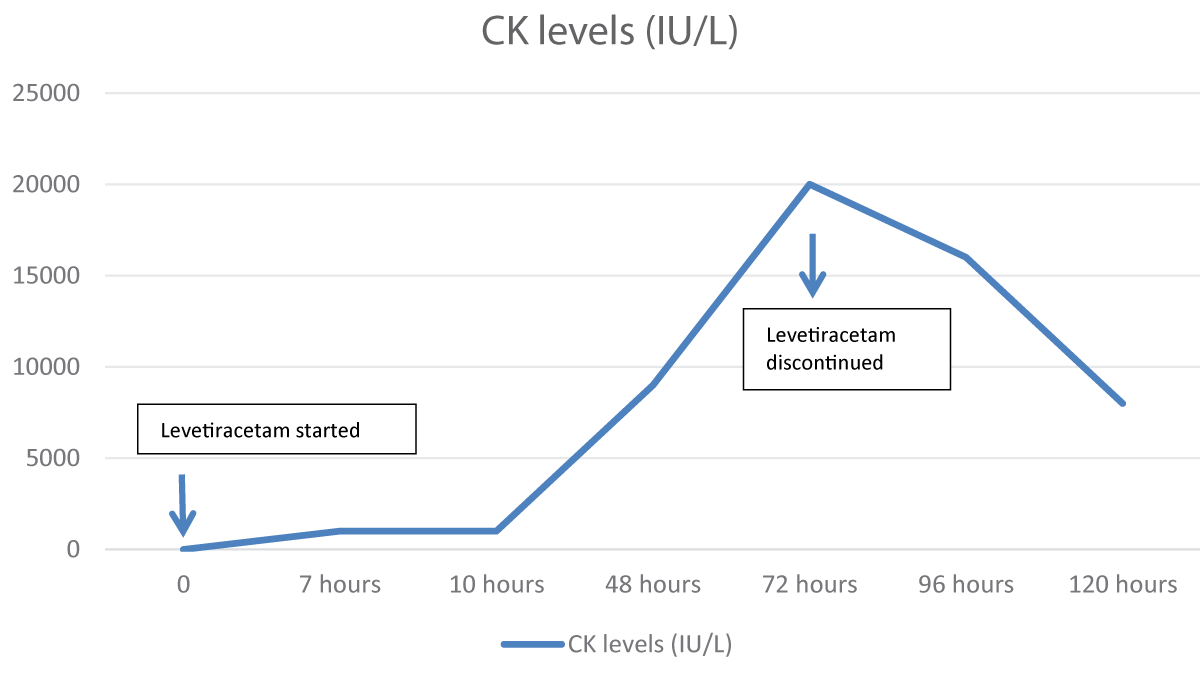 Figure 2: CK level trend in association with levetiracetam u...
Figure 2: CK level trend in association with levetiracetam u...
Moinuddin IA. Suspected Levetiracetam-Induced Rhabdomyolysis: A Case Report and Literature Review. Am J Case Rep. 2020 Oct 28;21:e926064. doi: 10.12659/AJCR.926064. PMID: 33112844; PMCID: PMC7603803.
Tiglis M, Hurmuzache T, Bologa C, Neagu TP, Mirea L, Grintescu IM. Rhabdomyolysis-Induced Acute Renal Injury in a Schizophrenic Patient. J Crit Care Med (Targu Mures). 2020 Nov 7;6(4):249-252. doi: 10.2478/jccm-2020-0032. PMID: 33200097; PMCID: PMC7648442.
Bosch X, Poch E, Grau JM. Rhabdomyolysis and acute kidney injury. N Engl J Med. 2009 Jul 2;361(1):62-72. doi: 10.1056/NEJMra0801327. Erratum in: N Engl J Med. 2011 May 19;364(20):1982. PMID: 19571284.
Torres PA, Helmstetter JA, Kaye AM, Kaye AD. Rhabdomyolysis: pathogenesis, diagnosis, and treatment. Ochsner J. 2015 Spring;15(1):58-69. PMID: 25829882; PMCID: PMC4365849.
Shahbaz N, Younus SM, Khan SA, Ain Q-, Khan MA, Memon MH. Levetiracetam Induced Increase in Creatine Phosphokinase Levels. J Coll Physicians Surg Pak. 2017 Mar;27(3):S63-S64. PMID: 28302251.
Akiyama H, Haga Y, Sasaki N, Yanagisawa T, Hasegawa Y. A case of rhabdomyolysis in which levetiracetam was suspected as the cause. Epilepsy Behav Case Rep. 2014 Sep 16;2:152-155. doi: 10.1016/j.ebcr.2014.08.001. PMID: 25667895; PMCID: PMC4308062.
Al-Quliti K, Alhujeily RM. Acute kidney injury with levetiracetam in patient with epilepsy: a case report. Case Reports in Clinical Medicine. 2022;11(3):48-54.
Di Lorenzo R, Li Y. Rhabdomyolysis associated with levetiracetam administration. Muscle Nerve. 2017 Jul;56(1):E1-E2. doi: 10.1002/mus.25548. Epub 2017 Mar 23. PMID: 28039868.
Abou-Khalil B. Levetiracetam in the treatment of epilepsy. Neuropsychiatr Dis Treat. 2008 Jun;4(3):507-523. doi: 10.2147/ndt.s2937. PMID: 18830435; PMCID: PMC2526377.
Carnovale C, Gentili M, Antoniazzi S, Clementi E, Radice S. Levetiracetam-induced rhabdomyolysis: Analysis of reports from the Food and Drug Administration's Adverse Event Reporting System database. Muscle Nerve. 2017 Dec;56(6):E176-E178. doi: 10.1002/mus.25972. Epub 2017 Sep 30. PMID: 28888059.
Kim H, Jo S, Park KW, Han SH, Lee SA. A Case of Phenytoin-induced Rhabdomyolysis in Status Epilepticus. J Epilepsy Res. 2016 Jun 30;6(1):36-38. doi: 10.14581/jer.16007. PMID: 27390679; PMCID: PMC4933680.
Prendergast BD, George CF. Drug-induced rhabdomyolysis--mechanisms and management. Postgrad Med J. 1993 May;69(811):333-336. doi: 10.1136/pgmj.69.811.333. PMID: 8393995; PMCID: PMC2399808.
Gunathilake R, Boyce LE, Knight AT. Pregabalin-associated rhabdomyolysis. Med J Aust. 2013;199(9):624-625.
Kazmi JS, Albarghouthy N, Ramsaywak R. Levetiracetam-Induced Rhabdomyolysis Reversed by Discontinuation: A Case Report. Cureus. 2023;15(11):e48955. doi: 10.7759/cureus.48955. PMID: 38111426; PMCID: PMC10726084.
Nizamudeen K, Sabu ST, Dharan SS. An overview of the rare and life-threatening adverse effects of levetiracetam. World Journal of Pharmaceutical Research. 9:649-670. DOI: 10.20959/wjpr202011-18761