
The Influence of Low Pesticide Doses on Fusarium Molds
Veterinary Science受け取った 01 Jul 2024 受け入れられた 16 Jul 2024 オンラインで公開された 17 Jul 2024
Focusing on Biology, Medicine and Engineering ISSN: 2995-8067 | Quick Google Scholar
Next Full Text
Harnessing Microbes for the Benefit of Man


受け取った 01 Jul 2024 受け入れられた 16 Jul 2024 オンラインで公開された 17 Jul 2024
The agricultural sector is a large consumer of synthetic chemical products, especially fertilizers and plant protection products. Therefore, an emerging concern nowadays is to reduce chemicals’ use in agriculture. One of the approaches is to reduce the doses of plant protection products, as much as possible, while keeping the treatments’ efficacy. The present work presents the antifungal action of three commercial plant protection products, tested at recommended as well as reduced doses, against important phytopathogenic molds of the Fusarium genus. In vitro, results have shown that two of the tested products could be used at reduced doses while keeping their antifungal activity. The commercial pesticide containing prothioconazole 53 g/L, spiroxamine 224 g/L, and tebuconazole 148 g/L mixture was able to inhibit completely the growth of three virulent F. culmorum strains, even when fungicide treatment was applied in 25% reduced dose. Lower efficacy was seen on F. graminearum strains, however, there were no significant differences (p < 0.05) between the commercially recommended dose and the 25% reduced dose. Another efficient pesticide in Fusarium control contains triadimenol 43 g/L, spiroxamine 250 g/L, and tebuconazole 167 g/L. Tested in a reduced dose (28.6% less than the commercial recommended dose) it completely inhibited the F. graminearum Fg183 (DSM 4527) strain and inhibited the growth of various F. culmorum strains with at least 97.50% efficacy. However, there are some fungal strains, such as the aggressive F. graminearum Fg96 strains that were less susceptible to pesticide treatments even at commercially recommended doses of fungicides.
Fusariosis is an extremely damaging disease for cereals and many other economically important plants. The infection can be installed in all vegetation stages and can continue also during storage, creating significant losses or reducing the yield quality. However, in wheat, the most damaging are the infections that occur during flowering. The Fusarium Head Blight (FHB) is highly detrimental to all cereals and could be caused by potentially mycotoxigenic molds of the genus Fusarium. The most common Fusarium species on wheat are F. graminearum and F. culmorum, which belong to the Discolor section []. Other Fusarium species are also mentioned, such as F. avenaceum, a member of the Roseum section [], as well as F. poae and F. langsethiae, both from the Sporotrichiella section [].
The economic losses are not limited to yield reduction. In many cases, the quality is also depreciated, making the harvest unfit for human consumption or animal feed, due to mycotoxin contamination. These toxins are secondary metabolites produced by the fungi, that can be accumulated in the crop. If certain contamination levels are reached within the harvest, the crop quality is depreciated, making the yield unsuitable for consumption []. Most of the Fusarium spp. contaminants in cereals are mycotoxin producers. Moreover, the Fusarium spp. mycotoxins are considered among the most dangerous toxins for human and animal health []. These fungal species are capable of producing three of the most important classes of mycotoxins: fumonisins (FB1, FB2, FB3), zearalenone, and trichothecenes, such as deoxynivalenol, nivalenol, HT-2 and T-2 toxins, diactoxyscripenol and monoacetoxyscripenol. Such fungi can also produce emerging mycotoxins, like fusoproliferin, beauvericin, eniatins, moniliformin, or other mycotoxins such as fusaric acid, fusarin AD, gliotoxin, butenolith, which are relatively recent discovered and less studied [].
Although ruminants and poultries are less sensitive than monogastric the deleterious effects of Fusarium mycotoxins can still occur, damaging the liver and kidney functions. Fusariotoxicoses on farm animals highly reduce their performance. Various acute or chronic symptoms/effects occur, depending on the intoxication levels. Immunosuppressive, hepatotoxic, nephrotoxic, as well as alteration of the reproductive function are some of the most evident side effects []. In humans, the Fusarium mycotoxins induce acute toxicity, although, carcinogenic effects are also considered []. The main risks of counteracting food contamination the crop production and food processing or improper storage [].
To prevent Fusarium spp. infections, several management practices can be considered, such as crop rotation, tolerant cultivars, and rational use of agrochemical inputs, as the most significant. There are many commercial plant protection products available on the market, some of which contain a mixture of different classes of active substances that make the product much more effective. Among the currently-used fungicides against FHB include tebuconazole, propiconazole, metconazole, picoxistrobin, trifloxystrobin, proquinazid, triadimenol, and many others []. However, studies have shown that strobilurin treatments, although effective against FHB, are inducing higher levels of DON mycotoxins []. Biological means can also improve plants’ health and prevent fungal infections []. On the European market, several active ingredients of biological origin are allowed. Against fusariosis, the most effective microbial strains belonging to Bacillus and Trichoderma genera.
The benefits of using plant protection products are undeniable in agricultural performance, as they prevent crop losses and improve the yield and quality of the harvest []. However, at the European level, there is a current demand to reduce the amount of chemical pesticides used in agriculture []. This is due to the negative side effects of pesticide residues on human and animal health, as well as on the environment []. One of the proposed measures is to reduce the doses of the active substances if efficacy is maintained []. Therefore, this study aimed to evaluate, in vitro, the antifungal action against Fusarium molds of some commercial pesticides in reduced doses compared to the recommended dose.
Three Commercial Fungicides (CF) were used in this study, encoded CF1-NP, CF2-F, and CF3-FP (Table 1). All these are chemical-based products available on the Romanian market and in other European countries. The CF1-NP product contains two active ingredients, while the other two (CF2-F and CF3-FP) fungicides contain three active substances. These three commercial pesticides were tested in vitro, in two doses. One of the tested doses was currently recommended to control fusariosis on cereals as presented on the product’s label, while the other was a reduced dose, as presented in the following table (Table 1). In the field, the recommended dose of the CF can be applied in 200 to 400 L of water per ha. This was extrapolated, for the in vitro study, as 130 µl of fungicide solution per plate of 9 cm diameter.
The antifungal activity of these products was evaluated against five plant pathogenic Fusarium spp. molds. The phytopathogenic fungi used in the laboratory trials were Fusarium culmorum FC 46, FC 1056, and FC 1471 strains, and F. graminearum FG 96 and FG 183 strains. The FG 183 strain is a reference strain, available in the German Collection of Microorganisms and Cell Cultures GmbH, under accession number DSM 4527.
The in vitro testing was performed on Potato Dextrose Agar. The fungicides were suspended in sterile distilled water (SDW) and plated on the medium. One of the tested doses was the application dose, currently recommended on the product label, while the other dose was reduced to 25%, in the case of CF1-NP and CF2-F, or 28.57% in the case of CF3-FP. As the test was performed in Petri plates of 9 cm diameter, the amounts of CF were adapted from L/ha to µl/Plate (Table 1). To ensure proper distribution of the fungicide on the substrate, the CF was resuspended in SDW, and 130 µl of the resulting solution was uniformly dispersed on top of the PDA medium using a Drigalski spreader. After infusion, the fungi were inoculated on top of the agar layer as mycelial plugs of 5 mm diameter. Control plates of each fungal pathogen were prepared, where fungi were grown on PDA with no pesticides.
The incubation was carried out at 25°C, while the biometric measurements of the fungal colony diameter were taken after 5, 7, and 10 days from inoculation. The inhibition efficacy of the pesticides against the fungi was calculated according to Lahlali and Hirji []. To evaluate the CF efficacy on fungal inhibition, control plates with no fungicide treatments were prepared. These control plates allowed fungal development in normal growth conditions. The inhibitory activity of the CF treatments, in currently recommended and reduced doses, was compared to these controls.
The mycelial growth of the fungi was comparatively measured after the incubation on PDA, and PDA supplemented with CF at recommended and reduced application dose for field treatments.
In the first 5 days of incubation, some of the fungal strains were completely inhibited by the presence of pesticides within the medium (Table 2). All F. culmorum strains were not able to grow in the presence of CF3-FP pesticide, at any of the tested doses, and on CF2-F pesticide in the recommended dose for field applications. Compared to the CF2-F and CF3-FP pesticides that are based on three active ingredients, the CF1-NP (based on trifloxystrobin 150 g/L and prothioconazole 175 g/L) was less effective. However, it substantially reduced the fungal growth, even in a reduced dose, compared to the control, where no fungicide was added to the substrate.
 Table 2: Mycelial growth of the fungal strains grown in fungicidal conditions compared to untreated controls after 5 days of incubation.
Table 2: Mycelial growth of the fungal strains grown in fungicidal conditions compared to untreated controls after 5 days of incubation.Among the studied fungal strains, Fg96 was less affected. However, even at a reduced application dose, the tested fungicides inhibited mycelial growth to less than half of the growth in the control plates.
Seven days after incubation the fungal strains continued to the same growing trend. Moreover, those strains completely inhibited by the presence of the fungicide maintained no growth after seven days of incubation on PDA supplemented with pesticides (Table 3).
 Table 3: Mycelial growth of the fungal strains grown in fungicidal conditions compared to untreated controls after 7 days of incubation.
Table 3: Mycelial growth of the fungal strains grown in fungicidal conditions compared to untreated controls after 7 days of incubation.Ten days after inoculation, new biometric measurements were made on the mycelial growth, and the efficacy of fungal growth inhibition was calculated. According to our data, the CF1-NP pesticide at the recommended application dose was less effective in fungal inhibition compared to the other two products, even when CF2-F and CF3-FP were tested at reduced doses (Table 4).
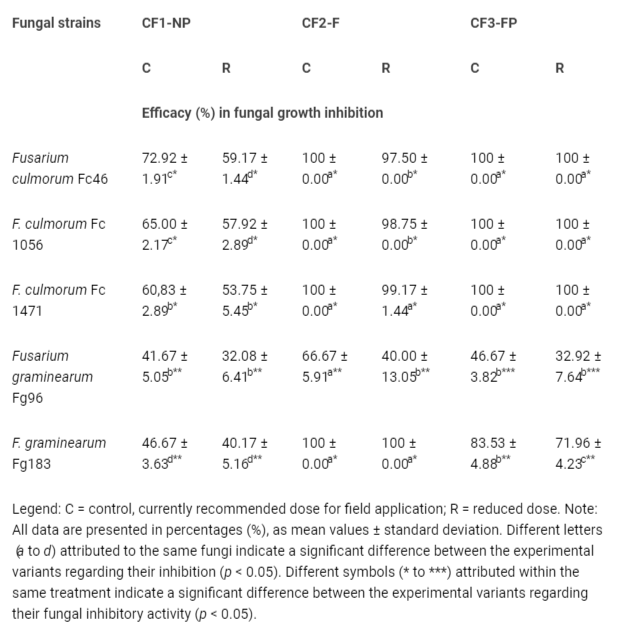 Table 4: Efficacy of fungal growth inhibition using different CF in reduced and currently recommended dose (after 10 days of incubation).
Table 4: Efficacy of fungal growth inhibition using different CF in reduced and currently recommended dose (after 10 days of incubation).Tested against various F.culmorum strains (FC 46, FC 1056, and FC 1471), the CF3-FP in both tested doses, and CF2-F in the currently recommended dose completely inhibited mycelial growth, compared to the untreated fungal control (Figure 1a-1c), while CF2-F in reduced dose inhibited the mycelial growth with at least 97.5% efficacy (Table 1).
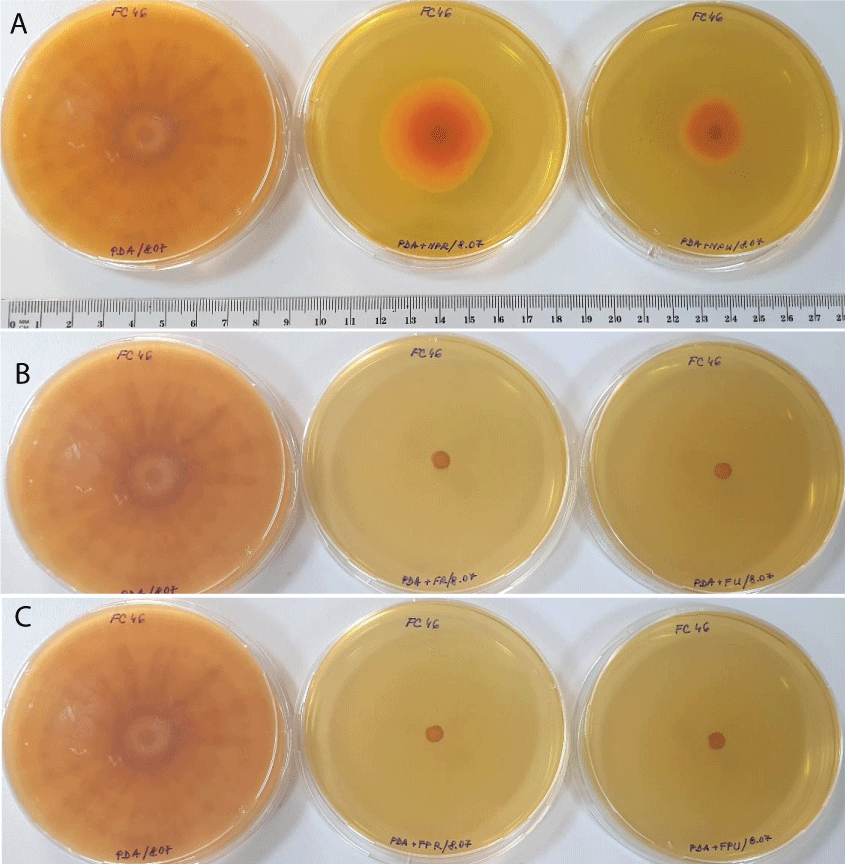 Figure 1: Fusarium culmorum Fc46 fungal growth in control plates (left) compared to CF1-NP (a), CF2-F (b), and CF3-FP (c) pesticide treatments in reduced (center) and currently recommended dose (right) (after 10 days of incubation).
Figure 1: Fusarium culmorum Fc46 fungal growth in control plates (left) compared to CF1-NP (a), CF2-F (b), and CF3-FP (c) pesticide treatments in reduced (center) and currently recommended dose (right) (after 10 days of incubation).The Fusarium graminearum Fg96 was the most tolerant strain to all pesticides used. The Fg96 developed mycelial growth in all pesticide-treated variants both in low dose, as well as in the recommended application dose (Figure 2).
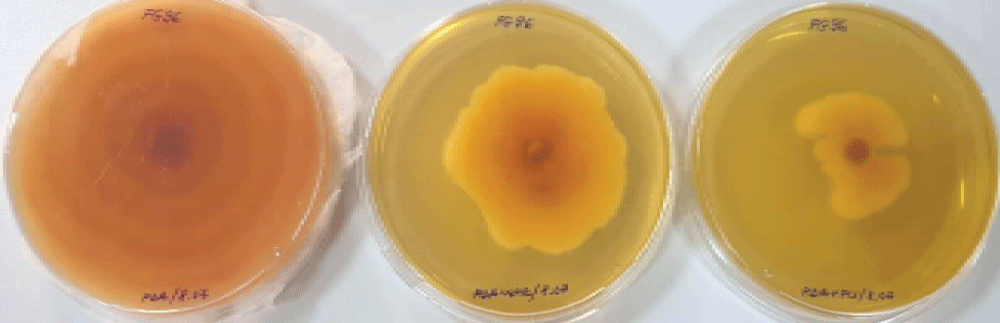 Figure 2: Fusarium graminearum Fg96 fungal growth in control plates (left) compared to CF1-NP treatments in reduced (center) and currently recommended dose (right), after 10 days of incubation.
Figure 2: Fusarium graminearum Fg96 fungal growth in control plates (left) compared to CF1-NP treatments in reduced (center) and currently recommended dose (right), after 10 days of incubation.Similar tests are mentioned to be performed on Fusarium spp. fungi isolated from infected wheat grains. Among the tested fungicides triazoles (prothioconazole 250 g/L and tebuconazole 251,2 g/L) and strobilurins (azoxystrobin 250 g/l and fluoxastrobin 480 g/l) were used in different concentrations. The fungi were grown on PDA supplemented with up to 100 mg/l commercial pesticides. Among the tested fungicides, prothioconazole was the most effective in reducing fungal growth, while tebuconazole proved to be efficient only in high concentration, against Fusarium crookwellense, F. tricinctum and F. culmorum. Isolates of F. tricinctum and F. graminearum were very responsive to fluoxastrobin treatment, but in general, the strobilurins showed a lower influence on the tested fusaria [].
Melchett [] considers that the negative impact of pesticides on biodiversity is underestimated. The decline of bee, butterfly, and partridges’ populations is associated with pesticide use []. Direct and indirect exposure can counteract various health issues depending on the pesticide type []. Organophosphates and carbamates affect the nervous system, while others irritate the skin or eyes [,]. Acute toxic effects occur immediately after exposure and have more evident effects, on both humans and animals []. More problematic those, are the chronic health issues, which appear after long-term, low-dose exposure to pesticides, and include various diseases and disorders, including cancers, reproductive dysfunctionalities, neurobehavioral disorders, impaired immune function, and allergic sensitization reactions []. Therefore, tightening pesticide regulation, reducing synthetic pesticide use, as well as biopesticide implementation should be considered. This follows the current integrated pest management strategies and the European strategies for plant protection [].
Studying the in vitro efficacy of three commercial fungicides against various Fusarium pathogens, it was revealed that F. culmorum and F. graminearum fungi were more sensitive to the CF2-F and CF3-FP pesticides, containing mixtures of three active ingredients (spiroxamine, tebuconazole, and triadimenol or prothioconazole, respectively), compared to CF1-NP pesticide, containing trifloxystrobin and prothioconazole. Although against various F. culmorum strains (FC46, FC 1056, and FC 1471), both CF2-F and CF3-FP pesticides provided a high inhibition efficacy when used in reduced dose (25% less PC3-FP, and 28.6% less PC2-F) they have a low efficacy against some F. graminearum pathogens (Fg 96 strain especially). Therefore, in the context of pesticide reduction in agriculture, the use of low pesticide doses cannot be considered a viable solution for plant protection. Other integrated pest management strategies should be considered.
Thrane U. Grouping Fusarium section Discolor isolates by statistical analysis of quantitative high-performance liquid chromatographic data on secondary metabolite production. J Microbiol Methods. 1990;12(1):23-39.
Benyon FHL, Burgess LW, Sharp PJ. Molecular genetic investigations and reclassification of Fusarium species in sections Fusarium and Roseum. Mycol Res. 2000;104:1164-1174.
Schmidt H, Adler A, Holst-Jensen A, Klemsdal SS, Logrieco A, Mach RL, Nirenberg HI, Thrane U, Torp M, Vogel RF, Yli-Mattila T, Niessen L. An integrated taxonomic study of Fusarium langsethiae, Fusarium poae and Fusarium sporotrichioides based on the use of composite datasets. Int J Food Microbiol. 2004 Sep 15;95(3):341-9. doi: 10.1016/j.ijfoodmicro.2003.12.012. PMID: 15337598.
Ferrigo D, Raiola A, Causin R. Fusarium Toxins in Cereals: Occurrence, Legislation, Factors Promoting the Appearance and Their Management. Molecules. 2016 May 13;21(5):627. doi: 10.3390/molecules21050627. PMID: 27187340; PMCID: PMC6274039.
Loiseau N, Polizzi A, Dupuy A, Therville N, Rakotonirainy M, Loy J, Viadere JL, Cossalter AM, Bailly JD, Puel O, Kolf-Clauw M, Bertrand-Michel J, Levade T, Guillou H, Oswald IP. New insights into the organ-specific adverse effects of fumonisin B1: comparison between lung and liver. Arch Toxicol. 2015 Sep;89(9):1619-29. doi: 10.1007/s00204-014-1323-6. Epub 2014 Aug 26. PMID: 25155190.
Stanciu O, Juan C, Miere D, Loghin F, Mañes J. Occurrence and co-occurrence of Fusarium mycotoxins in wheat grains and wheat flour from Romania. Food Control. 2017;73:147-155.
Mohamed AEE, Boiu-Sicuia OA, Cornea CP. Characterization of feed contamination by Fusarium sp. - A review. Sci Bull Ser F Biotechnol. 2022;XXVI(2):92-103.
Meneely J, Greer B, Kolawole O, Elliott C. T-2 and HT-2 Toxins: Toxicity, Occurrence and Analysis: A Review. Toxins (Basel). 2023 Jul 29;15(8):481. doi: 10.3390/toxins15080481. PMID: 37624238; PMCID: PMC10467144.
Qu Z, Ren X, Du Z, Hou J, Li Y, Yao Y, An Y. Fusariummycotoxins: The major food contaminants. mLife. 2024 May 13;3(2):176-206. doi: 10.1002/mlf2.12112. PMID: 38948146; PMCID: PMC11211685.
Chen A, Islam T, Ma Z. An integrated pest management program for managing fusarium head blight disease in cereals. J Integr Agric. 2022;21(12):3434-3444.
Amarasinghe CC, Tamburic-Ilincic L, Gilbert J, Babel AL, Dilantha Fernando WG. Evaluation of different fungicides for control of Fusarium head blight in wheat inoculated with 3ADON and 15ADON chemotypes of Fusarium graminearum in Canada. Can J Plant Pathol. 2013;35(2):200-208.
Tudi M, Li H, Li H, Wang L, Lyu J, Yang L, Tong S, Yu QJ, Ruan HD, Atabila A, Phung DT, Sadler R, Connell D. Exposure Routes and Health Risks Associated with Pesticide Application. Toxics. 2022 Jun 19;10(6):335. doi: 10.3390/toxics10060335. PMID: 35736943; PMCID: PMC9231402.
EUROPEAN COMMISSION. Farm to Fork Strategy: For a fair, healthy and environmentally-friendly food system. May 2020. Available from: https://ec.europa.eu/food/farm2fork_en#:~:text=The%20Farm%20to%20Fork%20Strategy%20is%20at%20the%20heart%20of,%2C%20healthy%20and%20environmentally%2Dfriendly.&text=The%20Farm%20to%20Fork%20Strategy%20aims%20to%20accelerate%20our%20transition,neutral%20or.
Kalyabina VP, Esimbekova EN, Kopylova KV, Kratasyuk VA. Pesticides: formulants, distribution pathways and effects on human health - a review. Toxicol Rep. 2021 Jun 6;8:1179-1192. doi: 10.1016/j.toxrep.2021.06.004. PMID: 34150527; PMCID: PMC8193068.
Lahlali R, Hijri M. Screening, identification and evaluation of potential biocontrol fungal endophytes against Rhizoctonia solani AG3 on potato plants. FEMS Microbiol Lett. 2010 Oct;311(2):152-9. doi: 10.1111/j.1574-6968.2010.02084.x. Epub 2010 Aug 25. PMID: 20738401.
Müllenborn C, Steiner U, Ludwig M, Oerke EC. Effect of fungicides on the complex of Fusarium species and saprophytic fungi colonizing wheat kernels. Eur J Plant Pathol. 2008;120:157-166.
Melchett P. Pesticides – experts ignore the most serious threat to UK wildlife. Biodivers. 2017;18(2-3):60-63.
Mdeni NL, Adeniji AO, Okoh AI, Okoh OO. Analytical Evaluation of Carbamate and Organophosphate Pesticides in Human and Environmental Matrices: A Review. Molecules. 2022 Jan 18;27(3):618. doi: 10.3390/molecules27030618. PMID: 35163876; PMCID: PMC8840499.
Goswami SK, Singh V, Chakdar H, Choudhary P. Harmful Effects of Fungicides-Current Status. IJAEB. 2018;11:1011-1019.
Ursan M, Boiu-Sicuia OA, Crăinescu II, Cornea CP. The Influence of Low Pesticide Doses on Fusarium Molds. IgMin Res. July 17, 2024; 2(7): 626-631. IgMin ID: igmin226; DOI: 10.61927/igmin226; Available at: igmin.link/p226
次のリンクを共有した人は、このコンテンツを読むことができます:
1University of Agronomic Sciences and Veterinary Medicine of Bucharest, Faculty of Biotechnology, 59 Mărăști Blvd, District 1, Bucharest, Romania
2Research – Development Institute for Plant Protection, 8 Ion Ionescu de la Brad Blvd, District 1, Bucharest, Romania
Address Correspondence:
Oana-Alina Boiu-Sicuia, University of Agronomic Sciences and Veterinary Medicine of Bucharest, Faculty of Biotechnology, 59 Mărăști Blvd, Research – Development Institute for Plant Protection, 8 Ion Ionescu de la Brad Blvd, District 1, Bucharest, Romania, Email: [email protected]
How to cite this article:
Ursan M, Boiu-Sicuia OA, Crăinescu II, Cornea CP. The Influence of Low Pesticide Doses on Fusarium Molds. IgMin Res. July 17, 2024; 2(7): 626-631. IgMin ID: igmin226; DOI: 10.61927/igmin226; Available at: igmin.link/p226
Copyright: © 2024 Ursan M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
 Figure 1: Fusarium culmorum Fc46 fungal growth in control pl...
Figure 1: Fusarium culmorum Fc46 fungal growth in control pl...
 Figure 2: Fusarium graminearum Fg96 fungal growth in control...
Figure 2: Fusarium graminearum Fg96 fungal growth in control...
 Table 1: Commercial fungicides....
Table 1: Commercial fungicides....
 Table 2: Mycelial growth of the fungal strains grown in fun...
Table 2: Mycelial growth of the fungal strains grown in fun...
 Table 3: Mycelial growth of the fungal strains grown in fun...
Table 3: Mycelial growth of the fungal strains grown in fun...
 Table 4: Efficacy of fungal growth inhibition using differe...
Table 4: Efficacy of fungal growth inhibition using differe...
Thrane U. Grouping Fusarium section Discolor isolates by statistical analysis of quantitative high-performance liquid chromatographic data on secondary metabolite production. J Microbiol Methods. 1990;12(1):23-39.
Benyon FHL, Burgess LW, Sharp PJ. Molecular genetic investigations and reclassification of Fusarium species in sections Fusarium and Roseum. Mycol Res. 2000;104:1164-1174.
Schmidt H, Adler A, Holst-Jensen A, Klemsdal SS, Logrieco A, Mach RL, Nirenberg HI, Thrane U, Torp M, Vogel RF, Yli-Mattila T, Niessen L. An integrated taxonomic study of Fusarium langsethiae, Fusarium poae and Fusarium sporotrichioides based on the use of composite datasets. Int J Food Microbiol. 2004 Sep 15;95(3):341-9. doi: 10.1016/j.ijfoodmicro.2003.12.012. PMID: 15337598.
Ferrigo D, Raiola A, Causin R. Fusarium Toxins in Cereals: Occurrence, Legislation, Factors Promoting the Appearance and Their Management. Molecules. 2016 May 13;21(5):627. doi: 10.3390/molecules21050627. PMID: 27187340; PMCID: PMC6274039.
Loiseau N, Polizzi A, Dupuy A, Therville N, Rakotonirainy M, Loy J, Viadere JL, Cossalter AM, Bailly JD, Puel O, Kolf-Clauw M, Bertrand-Michel J, Levade T, Guillou H, Oswald IP. New insights into the organ-specific adverse effects of fumonisin B1: comparison between lung and liver. Arch Toxicol. 2015 Sep;89(9):1619-29. doi: 10.1007/s00204-014-1323-6. Epub 2014 Aug 26. PMID: 25155190.
Stanciu O, Juan C, Miere D, Loghin F, Mañes J. Occurrence and co-occurrence of Fusarium mycotoxins in wheat grains and wheat flour from Romania. Food Control. 2017;73:147-155.
Mohamed AEE, Boiu-Sicuia OA, Cornea CP. Characterization of feed contamination by Fusarium sp. - A review. Sci Bull Ser F Biotechnol. 2022;XXVI(2):92-103.
Meneely J, Greer B, Kolawole O, Elliott C. T-2 and HT-2 Toxins: Toxicity, Occurrence and Analysis: A Review. Toxins (Basel). 2023 Jul 29;15(8):481. doi: 10.3390/toxins15080481. PMID: 37624238; PMCID: PMC10467144.
Qu Z, Ren X, Du Z, Hou J, Li Y, Yao Y, An Y. Fusariummycotoxins: The major food contaminants. mLife. 2024 May 13;3(2):176-206. doi: 10.1002/mlf2.12112. PMID: 38948146; PMCID: PMC11211685.
Chen A, Islam T, Ma Z. An integrated pest management program for managing fusarium head blight disease in cereals. J Integr Agric. 2022;21(12):3434-3444.
Amarasinghe CC, Tamburic-Ilincic L, Gilbert J, Babel AL, Dilantha Fernando WG. Evaluation of different fungicides for control of Fusarium head blight in wheat inoculated with 3ADON and 15ADON chemotypes of Fusarium graminearum in Canada. Can J Plant Pathol. 2013;35(2):200-208.
Tudi M, Li H, Li H, Wang L, Lyu J, Yang L, Tong S, Yu QJ, Ruan HD, Atabila A, Phung DT, Sadler R, Connell D. Exposure Routes and Health Risks Associated with Pesticide Application. Toxics. 2022 Jun 19;10(6):335. doi: 10.3390/toxics10060335. PMID: 35736943; PMCID: PMC9231402.
EUROPEAN COMMISSION. Farm to Fork Strategy: For a fair, healthy and environmentally-friendly food system. May 2020. Available from: https://ec.europa.eu/food/farm2fork_en#:~:text=The%20Farm%20to%20Fork%20Strategy%20is%20at%20the%20heart%20of,%2C%20healthy%20and%20environmentally%2Dfriendly.&text=The%20Farm%20to%20Fork%20Strategy%20aims%20to%20accelerate%20our%20transition,neutral%20or.
Kalyabina VP, Esimbekova EN, Kopylova KV, Kratasyuk VA. Pesticides: formulants, distribution pathways and effects on human health - a review. Toxicol Rep. 2021 Jun 6;8:1179-1192. doi: 10.1016/j.toxrep.2021.06.004. PMID: 34150527; PMCID: PMC8193068.
Lahlali R, Hijri M. Screening, identification and evaluation of potential biocontrol fungal endophytes against Rhizoctonia solani AG3 on potato plants. FEMS Microbiol Lett. 2010 Oct;311(2):152-9. doi: 10.1111/j.1574-6968.2010.02084.x. Epub 2010 Aug 25. PMID: 20738401.
Müllenborn C, Steiner U, Ludwig M, Oerke EC. Effect of fungicides on the complex of Fusarium species and saprophytic fungi colonizing wheat kernels. Eur J Plant Pathol. 2008;120:157-166.
Melchett P. Pesticides – experts ignore the most serious threat to UK wildlife. Biodivers. 2017;18(2-3):60-63.
Mdeni NL, Adeniji AO, Okoh AI, Okoh OO. Analytical Evaluation of Carbamate and Organophosphate Pesticides in Human and Environmental Matrices: A Review. Molecules. 2022 Jan 18;27(3):618. doi: 10.3390/molecules27030618. PMID: 35163876; PMCID: PMC8840499.
Goswami SK, Singh V, Chakdar H, Choudhary P. Harmful Effects of Fungicides-Current Status. IJAEB. 2018;11:1011-1019.