
Assessing Bee (Hymenoptera, Apoidea, Anthophila) Diversity and Floral Preference in Two Habitats in the Iberian Peninsula
Evolutionary Biology Ecosystem ScienceEnvironmental Sciences受け取った 18 Jun 2024 受け入れられた 01 Jul 2024 オンラインで公開された 02 Jul 2024
ISSN: 2995-8067 | Quick Google Scholar
Previous Full Text
Modeling of Cr3+ doped Cassiterite (SnO2) Single Crystals


受け取った 18 Jun 2024 受け入れられた 01 Jul 2024 オンラインで公開された 02 Jul 2024
The plant-pollinator relationship is one of the most investigated biological processes, not only because of its ecological importance (natural and farming ecosystems) but also its economic profitability (farming and biological products). Current losses of bee populations urge the need to assess the state of wild bee biodiversity in environments such as the Sierra de Guadarrama. Two characteristic sites with different plant diversities were compared by collecting bees using net trapping, a thicket, and a grassland. In this way, not only the possible influence of floral wealth on bee abundance was studied, but also the preference of these Hymenoptera towards any type of flower. Phenological patterns and predominant sex were also studied. 331 bee individuals, belonging to 6 families, 19 genera, and 46 species, were recorded in this study. Our results showed that bee diversity depends not only on environmental factors (temperature or plant composition and abundance) but biological as well (plant-pollinators matches or co-occurring species). Moreover, our study sets a starting point for debating the influence of managed bees (Apis mellifera) on wild bee communities. A preference for a small number of plant species (Cistus ladanifer, Echium vulgare, and Lavandula stoechas) was observed. In addition, there was a relationship between the type of corolla and the tongue length. Our study highlights the importance of this area of the Sierra de Guadarrama for wild bee biodiversity. All things considered, it falls on preserving those ecosystems with high floral wealth to favor the wild bee´s presence and its habitat in the foresight of climate change future scenarios.
Pollination constitutes one of the main biological processes, being essential for wild plant reproduction and providing several ecosystem services [-]. Since plant-insect interaction was known (105 million years ago), there has been a mutualistic direct relationship between angiosperms and pollinators []. Nowadays, although still debatable, almost 90% of angiosperm species in terrestrial ecosystems depend on animal pollination for their reproduction, insects are the most important ones [,].
Bees (Hymenoptera, Apoidea, Anthophila) are considered the most efficient group of pollinators among insects [,]. It has a high impact, with a global economic value averaging between US$235 and US$577 billion a year [,]. Bee pollinators perform key ecosystem services shown to enhance the quality and quantity of agricultural products in some world´s leading orchards [,]. Thus, bees are not only economically but also ecologically important for food production and terrestrial ecosystem survival [].
In Europe, 1,942 species of bees have been recorded [,]. The Iberian Peninsula is considered one of the hotspots for pollinator diversity with at least 1,097 bee species recorded [,] They are organized in two different groups, according to their tongue length [], and six different families: (i) Andrenidae (237 species in the Iberian Peninsula), Colletidae (87 species), Halictidae (202 species) and Melittidae (25 species) considered short-tongued bees; (ii) Apidae (304 species) and Megachilidae (242 species) considered long-tongued bees [,,]. The most diverse and common family of bees in the Iberian Peninsula is the Apidae which includes some of the most important managed pollinators, honey bee (Apis mellifera Linnaeus, 1758) and bumblebee species (Bombus Latreille, 1802), and other important genera as Anthophora Latreille, 1803 [,]. Another diverse family is the Andrenidae, with Andrena Fabricius, 1775 as the main representative genus []. Most bee families show solitary behavior or some level of aggregation (Andrenidae, Colletidae, Megachilidae, Melittidae) []. However, different degrees of sociality and social parasitism also occur (Apidae or Halictus Latreille, 1804) [,,,,]. Along the same lines, various foraging behaviors, monolectic (specialized in one genus), oligolectic (specialized in one family), and polylectic (specialized in several families of plants), can be found within these families [,].
Bees possess several morphological and physiological attributes that make them unique and very efficient pollinators. They present furry bodies and legs, with specialized structures to transport pollen, called corbicles or scopes [,]. They also have great flight abilities and specially designed mouth-parts, allowing them to easily access pollen and nectar [,]. These attributes change within the families, explaining the difference in the range of bee activity during the day and season, with some species being more active in the early spring (Andrena) and others in the early summer (Bombus) []. Moreover, these morphological and physiological attributes will define the types of habitats where the different bee species will be found [].
Nowadays, terrestrial ecosystems are facing a great loss of bee biodiversity [,,,]. Causes of this decline include introduced pathogens, invasive species, agricultural intensification, and changes in land management such as habitat conversion or incorrect use of pesticides [,,,]. The destruction of natural habitats has mainly been linked to anthropogenic activities causing a decrease in plant diversity and pollen availability, ultimately leading to the loss of pollinators´ communities and species [,,,,]. Moreover, bee communities are highly influenced by other external factors, including microclimatic conditions, biotic interactions among pollinators, or floral composition at local scales [,,]. The combination of different pollinator species, with different morphological and physiological traits, leads to an improvement of ecosystem functions and can compensate for negative effects caused by climatic and anthropogenic conditions [,,].
Previous studies have focused on arthropod pollinators in natural and managed ecosystems [,]. Although many bee species contribute to pollination, most research has concentrated on a limited number of these species that are highly correlated with economic profit, such as A. mellifera [,,]. Moreover, there is a lack of knowledge regarding variations in bee community composition along the day and the season []. Understanding the relationship between community composition, environmental factors, and seasonal changes is essential to prevent the decline of bee populations and their ecosystem services.
Considering these limitations and to minimize environmental impact, we used net trapping to study the role of wild bees in two different habitats, a grassland and a thicket, localized in the area of influence of Sierra de Guadarrama National Park, in Madrid. Because plant and bee diversity are related [,], we selected two specific habitats characterized by the mix of annual herbaceous in the grassland and the dominance of rockroses in the thicket. The differences in plant diversity between the habitats allowed us to study the variety and preferences of the bee community present in the area. We aimed to create an inventory of bee and plant communities in the area, which would lead to a better and more complete picture of arthropod communities in natural ecosystems. We also wanted to compare bee biodiversity in two different habitats to infer variation in bee communities concerning changes in plant composition. In addition, the circadian rhythms of these insects were compared aiming to identify activity peaks concerning the season, daily hour, and temperature.
We hypothesized that (i) higher bee biodiversity would be found in the grassland when compared to the thicket, (ii) higher bee activity would happen at mid-day in those months with higher temperatures (summer), (iii) there would be a preference towards flowers with open corollas, and (iv) meteorological conditions would highly influence our results.
The study took place in two different areas in Galapagar, a grassland and a thicket, next to the Sierra de Guadarrama National Park, in Madrid (Spain) (Appendix A. Table A.1). We surveyed two 25x25m plots, with approximately 200 m between them (Figure 1). Though distance between the sites was not bigger than mean foraging ranges of wild bees (< 500 m) [,], we considered that it was enough, due to their different floristic composition, to observed bee preference for certain plants within the same area and to get an approach of the bee community present. The two selected habitats were at a great enough distance from each other to avoid potential edge effects. The grassland is characterized by annual herbaceous and some woody plants such as Lamiaceae, Asteraceae, and Malvaceae. It is surrounded by rockroses (Cistus ladanifer L.) and small holm oaks (Quercus coccifera L.), and it has full sunlight. The thicket is characterized by rockroses, spurious holm oaks, and herbaceous plants. It is located at 400m from a residential area and is partially exposed to the sun.
The study was conducted for two consecutive years (2017-2019) and sampling took place two times every month. Each plot was randomly surveyed for one hour at three different times of the day: Morning (spring-summer from 7:00 - 9:00, and autumn-winter from 8:00 - 10:00), mid-day (13:00 - 15:00) and afternoon (spring-summer from 19:00 - 21:00, and autumn-winter from 16:00 - 18:00). Sampling followed variation in sun activity according to the season. Air temperature, meteorology, and plant species where bees were sampled were recorded. We considered the frequency of sampling was enough to provide a consistent and comprehensive dataset, without having too great of an impact on the local pollinator population. Moreover, sampling three times a day helped us capture the variation of daily activity patterns, giving us a complete picture of bee activity in our sampling sites. A two-year study period allowed us to observe long-term trends and the effects of year-to-year environmental changes on local bee populations, improving the robustness of the data and reducing the likelihood of short-term disturbances in the results. Thus, the criteria for selecting sampling sites and frequency ensured a comprehensive and representative study of bee populations across various environments.
All bee individuals were collected by aerial trapping using entomological nets. Captured bees were put in a jar with ethyl acetate, pinned with entomological pins, and stored in an entomological box for further conservation. The identification of the specimens was carried out using a stereo microscope, dichotomic keys and comparing materials from the Colección de Entomología from the Universidad Complutense de Madrid (UCME) and the Museo Nacional de Ciencias Naturales de Madrid, for each family or genera.
Randomization and standardization of samples have been suggested to accurately compare diversity metrics across sites as sample size generally varies across communities given equal effort []. Similarly, coverage has been suggested to measure how completely a community has been sampled []. In our study, we used sample-size-based rarefaction and extrapolation curves, with 95% confidence intervals, obtained by a bootstrap method and based on 400 replications, through R package iNEXT []. Thus, we assessed sampling completeness and evaluated the relationship between species richness and sampling effort [-]. Sampling completeness was assessed using bee species as taxonomic units and sites (grassland and thicket) as sampling effort units.
A complete biological assessment includes components of both richness and relative abundance of all species present in the area [,,]. Traditional diversity indexes, such as species richness or Shannon index are very sensitive to sampling effort and species relative abundance [,,]. Hill numbers have been suggested to overcome this problem [,]. However, our methodology was standardized, and the sample effort was the same in both sites. Therefore, we used the following indexes to measure biodiversity: (1) Species richness (S), which measures the total number of species in an assemblage, (2) Shannon diversity (H), which accounts for evenness on each site, (3) Margalef index (D), that evaluates species richness in a community independently of the pool size as it is based on the relationship between S and N (total number of individuals), (4) Jaccard index, that studies the similarity between two areas based on absence/presence data (qualitative data), and (5) Sorensen index, that is similar to Jaccard index but considers abundance data (quantitative data) [,,,]. Indexes analysis was performed by function specnumber and diversity from package vegan [] and function Hutcheson t-test from package ecolTest. Some indexes were performed manually as there was not any R package including these indexes.
For the statistical analysis, different variables were selected and compared with each other. Chi-squared tests and prop. test from the R package was used to determine the significance of each variable independently based on its Chi-square statistics and p - value []. Generalized Linear Models (GLM) were performed to test whether our variables (Family, Species, Month, and Plant) were predictors of the differences between the grassland and the thicket. We used a long-link function to account for binomial distribution in our data. All variables were analyzed independently and contrasted using obtained Estimated Marginal Means (EMMs). To test for plant composition influence, bees were divided into two groups according to length, (i) short-tongued bees (Andrenidae, Colletidae, Halictidae, and Melittidae) and (ii) long-tongued bees (Apidae and Megachilidae). Plants were separated according to the type of corolla, (i) open-corolla (Cistus ladanifer) or (ii) close-corolla (the rest of the plants). Chi-squared test was used to test for dependency between the type of corolla and the tongue length. The type of relationship between the variables was measured by an ODDS ratio and Risk estimate.
All these analyses and graphs were performed with package Stats [], emmeans [], DescTools [], fmbs [], and ggplot2 [-Díaz SM, Settele J, Brondízio E, Ngo H, Guèze M, Agard J, et al. The global assessment report on biodiversity and ecosystem services: Summary for policy makers. 2019.] from R software version 4.0 [].
A total of 333 individuals were collected through net sampling over two years (2017-2019). Two individuals were not identified to species level and thus were not included in further analysis. Therefore, 331 bee individuals belonging to 5 families, 19 genera, and 46 species constituted our final dataset (Table 1; Appendix A. Table A.2).
 Table 1: Bee composition was found across the grassland and the thicket in Galapagar from 2017 to 2019. Classification based on Ortiz-Sánchez [].
Table 1: Bee composition was found across the grassland and the thicket in Galapagar from 2017 to 2019. Classification based on Ortiz-Sánchez [].At the family level, Apidae (82%) was the most abundant family in our study, followed by Megachilidae (8%), Melittidae (5%), and Andrenidae (3%). Colletidae and Halictidae (1%) were the least abundant families (Appendix A. Table A.3).
Sites: In the grassland four out of six bee families were present (Apidae (89%), Megachilidae (9%), Andrenidae (1%) and Halictidae (1%)) (Appendix B. Figure B.1.). Contrary to the thicket, where all bee families were present (Apidae (54%), Melittidae (25%), Andrenidae (11%), Megachilidae (5%), Colletidae (3%) and Halictidae (2%)) (Appendix B. Figure B.1). Significantly more bees were recorded in the grassland (n = 270) than in the thicket (n = 61) (1-sample proportions test without continuity correction, x-squared = 131.97, df = 1, p –value = 2.2e-16, CI = 0.8 - 1.0, sample estimate = 0.8). It should be noted that this difference was still significant when honey bee individuals were removed from the analysis (1-sample proportions test without continuity correction, chi-squared = 44.3, df = 1, p - value = 1.4e-11, CI = 0.7-1.0, sample estimate = 0.8).
When sites were analyzed, Apidae and Megachilidae families seemed to explain differences across the grassland and the thicket (generalized linear model with binomial errors, intercept= -0.6, d.f. = 330, AIC = 250.4) being both families significantly more present in the grassland than in the thicket (EstimateApidae = 2.5, EstimateMegachildae = 2.6, p – values < 0.05) (Appendix C. Table C.1).
Sample completeness and biodiversity analysis: Sampling coverage (SC) indicated estimated values below complete sample adequacy (SC<1) in both the grassland (0.94) and the thicket (0.77) (Appendix A. Table A.4). The sample completeness curve showed that more individuals should have been sampled to assess total existing arthropod diversity in both sites (Appendix B. Figure B.2). However, our methodology was standardized, and equal effort was applied in both sites. Thus, observed values were used to compare arthropod biodiversity across sites. The most abundant species in both areas were Apis mellifera (57.4%), Bombus terrestris (8.2%), Anthophora bimaculate (5.4%), Dasypoda crassicornis (4.5%), and both Xylocopa cantabrita and Anthophora plumipes (2.7% respectively) (Appendix A. Table A.5; Appendix B. Figure B.3).
Species richness (SGrassland = 33; SThicket = 17) as well as Margalef index (DGrassland = 5.7; DThicket = 3.89) were higher in the grassland compared to the thicket. Estimated Shannon richness values (HGrassland = 1.8; HThicket = 1.75) were not significantly different between sites (Hutcheson t-test for two communities, Hutcheson t-statistic = 0.25, df = 107.7, p - value = 0.8). Both Jaccard (0.09) and Sorensen similarity indexes (0.37) were very close to 0 (Appendix A. Table A.4). Nevertheless, our results changed if honey bees (Apis mellifera) were removed from the analysis. Margalef indexes increased (DGrassland = 6.6; DThicket = 4.4) and estimated Shannon richness values (HGrassland = 2.76.; HThicket = 2.08) were significantly different between sites (Hutcheson t-test for two communities, Hutcheson t-statistic = 2.6, df = 46.4, p – value = 0.01). In the absence of A. mellifera, Jaccard´s index decreased (0.07) while Sorensen´s increased (0.44). Only four species were found in both sites (Andrena flavipes, Apis mellifera, Megachile leachella, and Megachile melanopyga) (Appendix A. Table A.5).
Although information on bee species was used to perform biodiversity indexes, the bee abundance of several species (e.g., Amegilla sp., Anthidium) was very low. Thus, further results will be discussed at the genus level, as bee representation at this level was higher.
The mean temperature in our sampling sites differed across the moment of the day and the month (Appendix B. Figure B.4). Higher temperatures (above 25 ºC) were observed at mid-day and afternoon in late spring and summer seasons (from May to September). The number of individuals recorded throughout the year significantly differed across months in both the grassland and the thicket (chi-squared test for given probabilities, chi-squared = 965.8, df = 11, p-value < 2.2e-16). Our results showed that more bee individuals were recorded in spring and early summer (May-July) at both sites (Figure 2).
Month seemed to influence differences across sites, with higher bee presence in April, May, and June in the grassland compared to thicket (generalized linear model with binomial errors, intercept = 0.8, d.f. = 330, AIC = 322.4, p – value < 0.05) (Appendix C. Table C.2). Individuals of some genera as Anthophora, Apis, Bombus or Megachile, were present from April to September while others were present only for one or two months earlier or later in the season (Anthidium, Ceratina, Colletes or Osmia) (Appendix B. Figure B.5).
Significant differences across the number of bees recorded were found depending on the moment of the day (chi-squared test for given probabilities, chi-squared = 165.7, df = 2, p - value < 2.2e-16). However, these differences were only significant when compared to the morning, as no significant differences were found between mid-day and afternoon (1-sample proportions test with continuity correction, chi-squared = 0.15, df = 1, p - value = 0.7, CI = 0.43-0.55). 63% of the genera were present at both times of the day (mid-day and afternoon), 11% preferred the afternoon while 26% had a preference for mid-day (Appendix A. Table A.6; Appendix B. Figure B.6).
Differences between sites were mainly due to the type of vegetation present, as other important factors, such as climate and altitude, were the same between the grassland and the thicket. Plant species richness was the same in the grassland when compared to the thicket (S = 8). However, flower availability and composition were different between the sites. The thicket had flowers available from March (Cistus ladanifer) while the grassland started to have flowers later in the season, around April (Diplotaxis tenuifolia (L.) DC., Ruta montana (L.)). However, the grassland had more plant species that flower during the warmer season (Appendix A. Table A.7). Some of the plant species were shared between both sites (Echium vulgare L., Daphne gnidium L. and Lavandula angustifolia). Others were only found in one site (Figure 2).
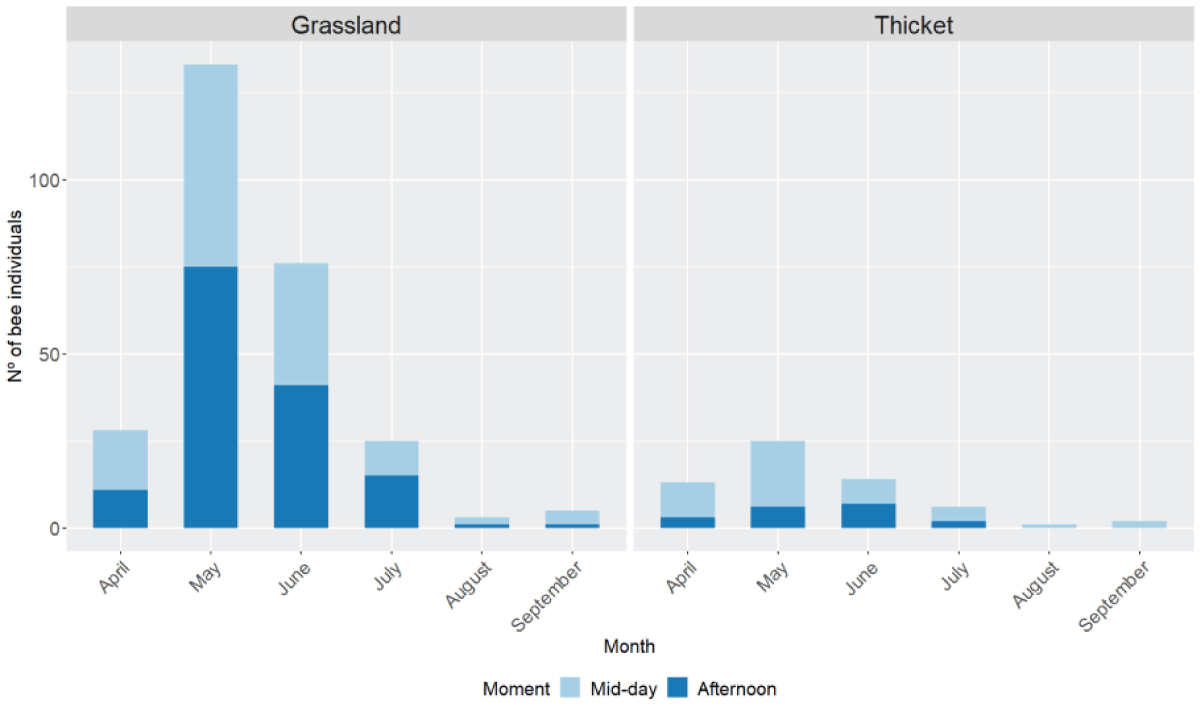 Figure 2: Number of bee individuals collected on each month (Month) and moment of the day (Moment) (mid-day (light blue, from 13:00 to15:00) and afternoon (dark blue, spring-summer from 19:00 - 21:00, and autumn-winter from 16:00 - 18:00)) at both sites, grassland (left) and thicket (right). From the 28th of June of 2017 until the 3rd of June of 2019.
Figure 2: Number of bee individuals collected on each month (Month) and moment of the day (Moment) (mid-day (light blue, from 13:00 to15:00) and afternoon (dark blue, spring-summer from 19:00 - 21:00, and autumn-winter from 16:00 - 18:00)) at both sites, grassland (left) and thicket (right). From the 28th of June of 2017 until the 3rd of June of 2019.Bee abundance was found to be significantly difference across plants (chi-squared test for given probabilities, chi-squared = 1,832.9, df = 12, p - value < 2.2e-16). C. ladanifer was the most visited species in the thicket (11%), while L. angustifolia prevailed in the grassland (69%) (Figure 3; Appendix A. Table A.8). Moreover, bee abundance across sites was found to be influenced by the plant species (generalized linear model with binomial errors, d.f. = 330, AIC = 71.3). Thus, higher bee presence in Lavandula angustifolia was recorded in the grassland compared to the thicket (generalized linear model with binomial errors, coefficientLavandula angustifolia = 5.4, p – value < 0.05) (Appendix C. Table C.3). Along the same lines, less bee presence in Echium vulgare was recorded in the grassland when compared to the thicket (generalized linear model with binomial errors, coefficientEchium Vulgare = -5.2, p – value < 0.05). In general, more bee genera visitedL. angustifolia (11), Echium vulgare (9), and Daphne gnidium (8) than other plants.
Regarding bee preferences, Apidae (Splant_visited = 11), Megachilidae (Splant_visited = 6), and Andrenidae (Splant_visited = 5) families visited more plant species than Halictidae (Splant_visited = 3), Melittidae (Splant_visited = 2) and Colletidae (Splant_visited = 1) (Appendix A. Table A.9; Appendix B. Figure B.7). At genus level, Apis (Splant_visited = 10), Andrena (Splant_visited = 5), Megachile (Splant_visited = 5), Xylocopa (Splant_visited = 4) and Anthidium (Splant_visited = 4) visited more plant species than the rest of the genera. Almost half of the genera (37%) visited only one plant species (Appendix A. Table A.9; Appendix B. Figure B.8).
Results showed that there was a significant relationship between the type of corolla (open or closed) and the type of tongue (short or long) (X-squared = 68.1, df = 1, p - value < 2.2e-16). Long-tongued bees were almost three times more likely to be found in flowers with close corollas than open corollas (odds. Ratio = 2.96, p - value < 2.2e-16) while short-tongued bees were two times more likely to be found in flowers with open corolla than long-tongued bees (risk ratio estimate, CI = 1.5-3.4, Estimate = 2.2, p – value < 2.2e-16).
A significantly greater number of female individuals (n = 284) were recorded in our study when compared to males (n = 47) (1-sample proportions test without continuity correction, chi-squared = 169.7, df = 1, p - value < 2.2e-16, CI = 0.8-1.0, sample estimates = 0.9) (Appendix B. Figure B.9). These results coincided in both the grassland (nfemales = 234, nmales = 36) and thicket (nfemales = 50, nmales = 11) (Appendix A. Table A.10). The phenology of both sexes matched, with both females and males being more active between April and June, and with lower activity in the warmer month (August) (Appendix B. Figure B.9).
Six bee families and forty-six bee species, previously recorded in the Iberian Peninsula [,], were detected in the considered areas. The study showed the influence of several environmental and biological factors on bee biodiversity, such as floral composition []. We further demonstrated the complementary use of different biodiversity indexes (E.g., species richness, Shannon and Jaccard index) to assess and compare two different habitats. Circadian rhythms showed bee preference towards the warmer part of the day (mid-day and afternoon) and late-spring season, characterized by sunny conditions and warm but mild temperatures. Both sexes preferred these conditions although females were more abundant than males at both sites.
According to Ortiz-Sánchez [] the bee fauna composition of the Iberian Peninsula is: Apidae (27.7%), Megachilidae (22.1%), Andrenidae (21.6%), Halictidae (18.4%), Colletidae (7.9%), and Melittidae (2.3%). These results matched ours. Besides Dasypoda, the most abundant genera (Apis, Anthophora, Bombus, and Xylocopa) were all polylectic bees (Falk, 2019). Dasypoda, considered an oligolectic bee genus, was found in two different plant species (Cistus ladanifer and Echium vulgare) matching with previous studies [,]. These results showed that the current study could establish an accurate picture of the potential community comprising this surrounding. Nevertheless, sample coverage showed incomplete sample size (SC<1) in both sites (Appendix A. Table A.5). Thus, higher and more intensive sampling effort (more days in the season) could have led to more complete results [].
The grassland showed higher bee richness than the thicket. However, species were not evenly distributed (H≈1.5) at either of the sites. This was a result of some species being more abundant than others in both the grassland (Apis mellifera, Bombus terrestris, and Anthophora bimaculata) and the thicket (A. mellifera and Dasypoda crassicornis). Nevertheless, index values changed in the absence of honey bees (A. mellifera). Biodiversity values (Shannon (H) and Margalef) increased in both the grassland and thicket. Along the same lines, Jaccard and Sorensen’s indexes supported that both sites differed in community composition. Firstly, Sorensen´s higher value in the absence of A. mellifera showed that differences in the number of individuals recorded between both sites were not as big as in its presence. Secondly, not only does bee composition differ between sites, with the grassland being more diverse than the thicket, but a few bee species dominated over the others in both sites. Nevertheless, traditional indexes are known to be sensitive to small sample sizes and rare species [,,,,].
Higher numbers of workers and female bees compared to males at both sites could be due to the biological functionality of each sex. While males’ function is to delimitate their territory and forage for themselves, females have to provide food for their offspring, and their main task is foraging [,]. This is why female bees are easier to find at any time in areas with high plant biodiversity, as they need more resources [].
Meteorological conditions and temperature fluctuations (environmental factors) have been shown to shorten bee activity periods, which are usually longer under warm and sunny conditions [,,]. Negative interactions across pollinators (biological factors) in areas with limited resources have also been proven to modify bee activity in natural landscapes [,]. Below, we discuss some environmental and biological factors that might have played an important role in shaping our biodiversity data and results.
Climatic conditions and temperature are known to modify bee activity in the field as temperature in their surroundings determines foraging activity [,,,]. The Iberian Peninsula, especially the Mediterranean area, is characterized by fluctuations in weather and temperature conditions, whose effects on pollinator rhythms have become more noticeable by climate change [,,]. Bee activity increases around 19 ºC, being very low at temperatures below 13 ºC depending on the bee species [,]. Our results showed that bee activity was higher in the warmer season (May-July) and in the temperate part of the day (mid-day and afternoon). Higher and lower temperatures, and adverse conditions after July, could explain the lower number of individuals recorded in the following months []. Some bee species are more resistant to colder periods and adverse climatic conditions [,,]. Our results supported this hypothesis with genera such as Bombus or Megachile, being active for longer periods and earlier in the season (Appendix B. Figure B.5) [,,,,]. Changes in pollinator rhythms have been observed in some species, such as A. mellifera, whose activity started earlier and ended earlier in the season, probably due to warmer days (Appendix B. Figure B.5) []. This could reflect the fact that climate change modifies bees foraging activity which may lead to other problems such as an increase in resource competition among different bee species due to niche overlap [,,,,].
High temperatures and adverse weather conditions not only limit bee activity but can also negatively influence floral initiation and pollen production [,]. Floral resources decline in late spring when most pollinator species start to be active, and therefore, floral resources are more likely to become a limiting factor [,]. Plant composition, floral abundance, and sunlight access were different between our sampling sites. Although Lavandula angustifolia was the predominant plant species in the grassland, other species such as Echium vulgare or Daphne gnidium were also common. On the other hand, Cistus ladanifer comprised the higher floral density in the thicket, with a low abundance of the other plants. L. angustifolia flowers are available from May to June while C. ladanifer has accessible flowers earlier in the season (March-June) (Appendix A. Table A.7). Thus, higher bee species richness and abundance present in the grassland could be due to higher flower availability (plant richness and abundance) when temperatures were higher, and bees were more active (May-June). This supported previous studies where bee activity, although synchronized with plant flowering, was highly influenced by climatic conditions, temperature, and plant abundance [,,,,]. Moreover, our results could support future climate change perspectives with flowering periods being modified, and plant-pollinator synchronization changing. This scenario might result in less efficient pollination and a shorter life span [,,,,].
Bee presence and plant preference are marked by several factors, not only resource availability but also by interspecific competition, local specialization, or biological traits [,,].
Our results showed high numbers of honey bees (Apis mellifera) (160 individuals out of 331). Honey bees are widespread managed bees essential for pollinator-dependent crops (E.g., apples, almonds) [,]. According to the Registro General de Explotaciones Agrarias (REGA), 32,845 hives were registered in Spain in 2019. Thus, the high numbers of honey bees in our study were probably a result of the presence of domestic honey bee hives in the surroundings. Several studies have shown a possible competition between wild bees and managed bees [,,,]. Moreover, floral resources could become a limiting factor when plant species richness and abundance are low [,,,]. Our results showed how A. mellifera was mainly found in Lavandula angustifolia and Cistus ladanifer, both dominant plants in the grassland and thicket respectively. Only a few bees were recorded in other plant species such as Echium vulgare or Daphne gnidium. One explanation could be that the presence of honey bees in the predominant plants might have displaced native wild bees towards other plants and areas, where resources were available, resulting in a smaller number of wild bee individuals recorded [,]. Along the same lines, our results showed how several genera such as Amegilla, Ceratina, Coelioxys, or Colletes, were more abundant in those months in which A. mellifera was less active (July-September) (Appendix B. Figure B.5). Higher resistance of wild bees when temperatures are below or over honey bee standards (from July-April) might have resulted in more wild bees recorded [,,]. A decrease in honey bee activity during those months might have led other generalist and specialistic bees to have access to more floral resources previously limited by the presence of A. mellifera [,,,]. These observations suggest how the foraging preferences of bee species and climatic conditions play a role in their presence throughout the day, the month, and the year, shaping the different ecological niches that bee species occupy.
Differences in sizes could have also played a role in our study as it has been shown to influence the capacity of bees to obtain nectar and pollen [,]. Bigger bees, such as Apis¸ Bombus, Xylocopa, or Anthophora, or territorial bees such as Rhodanthidium might have ruled over the smallest bees (Ceratina or Halictus), controlling the resources and displacing them towards other environments [-]. Interspecific competition could also happen between bees and other insects that might negatively affect bee presence []. Such an example is C. ladanifer, which is visited by other pollinators (Coleoptera and Diptera) and non-pollinators (Thomisidae) that can compete for floral resources with bees [,,].
Bee preference towards specific flowers is key to understanding foraging behavior and bee biodiversity in natural ecosystems [,,,]. Bees can visit flowers for pollen, nectar, or both, whose specialization depends not only on morphological traits (hairiness, proboscis length, or body size) but also on the quality of the floral resources and the accessibility of the floral structures (e.g. open or closed corollas) [,,,,,,]. This could be the reason why L. angustifolia was visited by 84.8% of individuals as it is known to constitute an easily accessible and primary nectar source for bee species such as honey bees and bumble bees [-]. A similar explanation can be used to explain why C. ladanifer was very popular in the thicket in comparison with other plants (62%). Several studies showed C. ladanifer is very popular among bees not only because of their pollen availability but also because of the very accessible big corolla [,,].
Although they forage in several plants, polylectic bees also have a preference towards specific families and may locally restrict visits to one or a few plants [,,,]. This was supported by our results with more than half of the bee genera visiting only one or two plant species (Appendix B. Figure B.8). Specialist bees are more vulnerable to plant richness decreased [,]. The low numbers of monolectic bees (Colletidae or Halictidae) in our study may be due to the higher abundance of a few plant species that were not attractive to these bees. However, we did not record enough wild bees to establish a preference towards specific plant species. Thus, more specific research should be done to test this hypothesis.
Tongue length has been associated with the foraging efficiency of bees and bee preference towards specific plants [,]. This was supported by our results as short-tongued bees rarely obtained pollen from flowers where nectar had difficult access but from flowers with more exposed pollen and nectar, like C. ladanifer. On the other hand, long-tongued bees preferred flowers with closer corollas such as L. angustifolia, D. gnidium, and E. vulgare. []. It has also been shown that long-tongued pollinators can pollinate flowers with open or closed corollas, while short-tongued pollinators are only able to pollinate close corolla flowers [,].
In addition, future studies could include more sampling days within each month and more replicates to avoid sampling bias and to follow these trends more accurately. Along the same lines, a more complete floral assessment of not only the area where we were sampling but also the close surroundings, could help us to better understand the bee community. Heterogenic landscapes have been positively linked to bee biodiversity and ecosystem services [,,,]. Understanding the biological relations across individuals within the environment and their circadian rhythms is key to understanding community composition [,]. A wider fauna assessment of the sites, including not only bee insects but also other arthropods, could help us understand potential interactions within our sites. This study provides a framework that can be implemented in other regions with similar ecological and geographical characteristics, enhancing bee conservation and habitat protection.
To conclude, wild pollinators are essential to maintain healthy and resilient ecosystems [,,,]. Moreover, pollinator biodiversity is globally declining as a response to anthropogenic activities and most of the bee species are threatened by climate change [,,]. Therefore, more assessments of bee diversity and phenology may be useful in designing appropriate conservation strategies. Our study constituted an updated list of wild bees present in this area of the Community of Madrid. It manifested that pollinator´s diversity and presence constitute a multifactor problem that includes both environmental and biological factors. The current research could outline significant implications for both global and regional bee conservation efforts illuminating the importance of protecting the natural landscapes to preserve local bee communities as well as the impact of managed species, such as A. mellifera, in pollinator communities. Future regional and global conservation policies should consider the importance of diverse and resilient floral habitats, in the foresight of climate change future scenarios [,,]. Encouraging the protection of native and diverse flowering plants providing food for a variety of wild bee species and preserving natural areas offering continuous blooms throughout the year, helping maintain pollinator communities.
The current study highlighted the importance of the floral diversity for holding diverse bee communities as well as pointing out that extreme temperatures are not suitable for bee activities. Moreover, we suggested possible negative interaction with A. mellifera whose presence might have led to resource depletion and domination forcing wild bees to find other foraging places outside our sampling sites and their usual activity period. Although greater efforts will lead to greater accuracy in results, we have set a starting point for future research toward a better understanding of pollinator communities and networks in natural environments in the Iberian Peninsula.
README- This file contains a summary of all the appendices contained within this folder.
Appendix A- Tables A.1 to A.10
Appendix C- Table C.1 to Table C.3
The research was designed to ensure that any negative effects on the environment were minimized. Efforts were made to ensure minimal stress and harm to the bees. Data was collected, analyzed, and reported honestly, ensuring the reliability of the findings. This study was conducted with a focus on providing new knowledge about pollinators’ biodiversity and conservation. No additional collaborations were involved. Necessary permits were obtained from the Sierra de Guadarrama National Park for conducting research with wildlife species in this area.
NG and CO designed the research; NG collected samples; NG and CO performed identification work; NG did the bioinformatic and statistical analysis; NG made the Figures and wrote the paper with input from CO. All authors contributed to the final version of the submitted manuscript.
Sampling was allowed by the pertinent permits from the Dirección General de Medio Ambiente de la Comunidad de Madrid, the Universidad Complutense de Madrid (UCM), and samples are stored in the Colección de Entomología de la UCM (UCME).
For their support with sampling and statistical analysis we would like to thank David Escobar, Pablo Castro, and Daniel Romero.
Fründ J, Linsenmair KE, Blüthgen N. Pollinator diversity and specialization in relation to flower diversity. Oikos. 2010;119(10):1581-1590. doi:10.1111/j.1600-0706.2010.18450.x
Nieto A, Roberts S, Kemp J, Rasmont P, Kuhlmann M, García Criado M, et al. European Red List of bees. Luxembourg: Publication Office of the European Union; 2014. 84 p.
Food and Agriculture Organization (FAO). Es hora de apreciar la labor de los polinizadores. https://www.fao.org/fao-stories/article/es/c/1129811/. Published 2018. Accessed March 25, 2024.
Díaz SM, Settele J, Brondízio E, Ngo H, Guèze M, Agard J, et al. The global assessment report on biodiversity and ecosystem services: Summary for policy makers. 2019.
Cardoso P, Barton PS, Birkhofer K, Chichorro F, Deacon C, Fartmann T, et al. Scientists' warning to humanity on insect extinctions. Biol Conserv. 2020;242. doi:10.1016/j.biocon.2020.108426
Ortiz-Sánchez FJ, Aguado Martín LÓ, Ornosa Gallego C. Bee diversity in Spain. Population trend and conservation measures (Hymenoptera, Apoidea, Anthophila). Ecosistemas. 2018;27(2):3-8. doi:10.7818/ecos.1315
Smith Pardo AH. Las abejas de Porce familia colletidae (hymenoptera: apoidea) notas y claves para los géneros presentes en la zona de influencia del embalse Porce ii. Rev Fac Nac Agron Medellín. 1999.
Klein A-M. Plant-pollinator interactions in changing environment. Basic Appl Ecol. 2011;12(4):279-341.
Ollerton J, Winfree R, Tarrant S. How many flowering plants are pollinated by animals? Oikos. 2011;120(3):321-326. doi:10.1111/j.1600-0706.2010.18644.x
Pardo A, Borges PA. Worldwide importance of insect pollination in apple orchards: A review. Agric Ecosyst Environ. 2020;293:106839.
Müller A, Diener S, Schnyder S, Stutz K, Sedivy C, Dorn S. Quantitative pollen requirements of solitary bees: implications for bee conservation and the evolution of bee–flower relationships. Biol Conserv. 2006;130(4):604-615.
Michener CD. The Bees of the World. 2nd ed. Baltimore: John Hopkins University Press; 2007.
Fründ J, Dormann CF, Holzschuh A, Tscharntke T. Bee diversity effects on pollination depend on functional complementarity and niche shifts. Ecology. 2013 Sep;94(9):2042-54. doi: 10.1890/12-1620.1. PMID: 24279275.
Leonhardt SD, Gallai N, Garibaldi LA, Kuhlmann M, Klein A-M. Economic gain, stability of pollination and bee diversity decrease from southern to northern Europe. Basic Appl Ecol. 2013;14(6):461-471.
Martínez-Puc JF, Merlo-Maydana FE. Importance of diversity of bees (Hymenoptera: Apoidea) and threats facing the tropical ecosystem of Yucatan, Mexico. J Selva Andina Anim Sci. 2014;1(2):28-34.
Khalifa SAM, Elshafiey EH, Shetaia AA, El-Wahed AAA, Algethami AF, Musharraf SG, AlAjmi MF, Zhao C, Masry SHD, Abdel-Daim MM, Halabi MF, Kai G, Al Naggar Y, Bishr M, Diab MAM, El-Seedi HR. Overview of Bee Pollination and Its Economic Value for Crop Production. Insects. 2021 Jul 31;12(8):688. doi: 10.3390/insects12080688. PMID: 34442255; PMCID: PMC8396518.
Quinet M, Warzée M, Vanderplanck M, Michez D, Lognay G, Jacquemart A-L. Do floral resources influence pollination rates and subsequent fruit set in pear (Pyrus communis L.) and apple (Malus x domestica Borkh) cultivars? Eur J Agron. 2016;77:59-69.
Reverté S, Bosch J, Arnan X, Roslin T, Stefanescu C, Calleja JA, et al. Spatial variability in a plant–pollinator community across a continuous habitat: high heterogeneity in the face of apparent uniformity. Ecography. 2019;42(9):1558-1568. doi:10.1111/ecog.04498.
Nunes-Silva P, Witter S, da Rosa JM, Halinski R, Schlemmer LM, Arioli CJ, Ramos JD, Botton M, Blochtein B. Diversity of Floral Visitors in Apple Orchards: Influence on Fruit Characteristics Depends on Apple Cultivar. Neotrop Entomol. 2020 Aug;49(4):511-524. doi: 10.1007/s13744-020-00762-1. Epub 2020 Mar 11. PMID: 32162245.
Ortiz-Sánchez FJ. Updated list of bee species in Spain (Hymenoptera: Apoidea: Apiformes). SEA Bowl. 2011;(49):265-281.
Álvarez Fidalgo P, Álvarez Fidalgo M, Noval Fonseca N, Castro L. Faunistic data on bees from the provinces of Asturias and León (northwest Spain), with a species not yet recorded in the Iberian Peninsula (Hymenoptera, Apoidea, Anthophila). Bol Asoc Esp Entomol. 2020;44.
Ortiz-Sánchez FJ. Iberian Fauna Checklist. Anthophila series (Insecta: Hymenoptera: Apoidea) in the Iberian Peninsula and Balearic Islands, 2020 ed. In: Ramos MA, Sánchez Ruiz M, eds. Iberian Fauna Documents. Madrid: National Museum of Natural Sciences, CSI; 2020. Vol. 14, pp. 83.
Ortiz-Sánchez F, del Castillo CR, Nieves-Aldrey J. Abundancia, Diversity and seasonal variation of apoidean genera (Hymenoptera, Apoidea) in two natural enclaves of the Community of Madrid (central Spain). Soc Entomol Aragonese Bowl. 2006;1(38):247-259.
Falk S. Field guide to the bees of Great Britain and Ireland. London: Bloomsbury Publishing; 2019.
Ornosa C, Ortiz-Sánchez FJ. Hymenoptera, Apoidea I. In: Ramos MA, et al., eds. Fauna Iberica. Madrid: Museo Nacional de Ciencias Naturales, CSIC; 2004. Vol. 23, pp. 556.
Dardón MJ, Torres F, Ornosa C. The subgenus Andrena (Micrandrena) (Hymenoptera: Andrenidae) in the Iberian Peninsula. Zootaxa. 2014 Oct 13;3872(5):467-97. doi: 10.11646/zootaxa.3872.5.3. PMID: 25544097.
Ortiz-Sánchez F, Ornosa C, Kuhlmann M. Identification keys for the Iberian species of the genus Colletes Latreille, 1802 (Hymenoptera, Colletidae). Zool Baetica. 2004;15:3-37.
Turley NE, Biddinger DJ, Joshi NK, López-Uribe MM. Six years of wild bee monitoring shows changes in biodiversity within and across years and declines in abundance. Ecol Evol. 2022 Aug 12;12(8):e9190. doi: 10.1002/ece3.9190. PMID: 35983174; PMCID: PMC9374588.
Gérard M, Vanderplanck M, Wood T, Michez D. Global warming and plant-pollinator mismatches. Emerg Top Life Sci. 2020 Jul 2;4(1):77-86. doi: 10.1042/ETLS20190139. PMID: 32558904; PMCID: PMC7326340.
Zattara EE, Aizen MA. Worldwide occurrence records suggest a global decline in bee species richness. One Earth. 2021;4(1):114-123. doi:10.1016/j.oneear.2020.12.005
Goulson D. Pesticides, Corporate Irresponsibility, and the Fate of Our Planet. One Earth. 2020;2(4):302-305. doi:10.1016/j.oneear.2020.03.004
Scheper J, Reemer M, van Kats R, Ozinga WA, van der Linden GT, Schaminée JH, Siepel H, Kleijn D. Museum specimens reveal loss of pollen host plants as key factor driving wild bee decline in The Netherlands. Proc Natl Acad Sci U S A. 2014 Dec 9;111(49):17552-7. doi: 10.1073/pnas.1412973111. Epub 2014 Nov 24. PMID: 25422416; PMCID: PMC4267333.
Klein AM, Vaissière BE, Cane JH, Steffan-Dewenter I, Cunningham SA, Kremen C, Tscharntke T. Importance of pollinators in changing landscapes for world crops. Proc Biol Sci. 2007 Feb 7;274(1608):303-13. doi: 10.1098/rspb.2006.3721. PMID: 17164193; PMCID: PMC1702377.
Joshi NK, Otieno M, Rajotte EG, Fleischer SJ, Biddinger DJ. Proximity to woodland and landscape structure drives pollinator visitation in apple orchard ecosystem. Front Ecol Evol. 2016;4:38.
Colla SR. The potential consequences of 'bee washing' on wild bee health and conservation. Int J Parasitol Parasites Wildl. 2022 Apr 2;18:30-32. doi: 10.1016/j.ijppaw.2022.03.011. PMID: 35399591; PMCID: PMC8989764.
Zurbuchen A, Landert L, Klaiber J, Müller A, Hein S, Dorn S. Maximum Foraging Ranges in Solitary Bees: Only Few Individuals have the Capability to Cover Long Foraging Distances. Biol Conserv. 2010;143:669-676. doi:10.1016/j.biocon.2009.12.003.
Kendall LK, Mola JM, Portman ZM, Cariveau DP, Smith HG, Bartomeus I. The potential and realized foraging movements of bees are differentially determined by body size and sociality. Ecology. 2022 Nov;103(11):e3809. doi: 10.1002/ecy.3809. Epub 2022 Sep 1. PMID: 35792515; PMCID: PMC9786665.
Roswell M, Dushoff J, Winfree R. A conceptual guide to measuring species diversity. Oikos. 2021;130(3):321-338. doi:10.1111/oik.07202
Chao A, Gotelli NJ, Hsieh T, Sander EL, Ma K, Colwell RK, et al. Rarefaction and extrapolation with Hill numbers: a framework for sampling and estimation in species diversity studies. Ecol Monogr. 2014;84(1):45-67.
Gotelli NJ, Chao A. Measuring and estimating species richness, species diversity, and biotic similarity from sampling data. In: Levin SA, ed. Encyclopedia of Biodiversity. 2nd ed. Vol. 5. Oxford: Academic Press; 2013; 195-211.
Russo L, Park M, Gibbs J, Danforth B. The challenge of accurately documenting bee species richness in agroecosystems: bee diversity in eastern apple orchards. Ecol Evol. 2015 Sep;5(17):3531-40. doi: 10.1002/ece3.1582. Epub 2015 Aug 5. PMID: 26380684; PMCID: PMC4567859.
Hsieh T, Ma K, Chao A. iNEXT: an R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol Evol. 2016;7(12):1451-1456.
Chafee M, Fernàndez-Guerra A, Buttigieg PL, Gerdts G, Eren AM, Teeling H, Amann RI. Recurrent patterns of microdiversity in a temperate coastal marine environment. ISME J. 2018 Jan;12(1):237-252. doi: 10.1038/ismej.2017.165. Epub 2017 Oct 24. PMID: 29064479; PMCID: PMC5739018.
Chao A, Kubota Y, Zelený D, Chiu CH, Li CF, Kusumoto B, et al. Quantifying sample completeness and comparing diversities among assemblages. Ecol Res. 2020;35(2):292-314.
Magurran AE. Diversidad ecológica y su medición. Barcelona: Ediciones Vedra; 1989.
Higes M, Martín R, Meana A. Nosema ceranae, a new microsporidian parasite in honeybees in Europe. J Invertebr Pathol. 2006 Jun;92(2):93-5. doi: 10.1016/j.jip.2006.02.005. Epub 2006 Mar 29. PMID: 16574143.
Yeom DJ, Kim JH. Comparative evaluation of species diversity indices in the natural deciduous forest of Mt. Jeombong. For Sci Technol. 2011;7(2):68-74. doi:10.1080/21580103.2011.573940.
Supriatna J. Biodiversity Indexes: Value and evaluation purposes. Paper presented at: E3S Web of Conferences; 2018. doi:10.1051/e3sconf/20184801001
Nisa RU, Gupta K, Wani SM, Allie KA, Kouser N. A study on diversity and ecology of ichthyofauna of Rajouri district, Jammu and Kashmir, India. Rec Zool Surv India. 2021;120(4):363-372.
Oksanen J, Blanchet FG, Guillaume Guénard, Friendly M, Kindt R, Legendre P, et al. vegan: Community Ecology Package (Version R package version 2.5-7), November 2020. Available from: https://CRAN.R-project.org/package=vegan
Lenth RV, Singmann H, Love J, Buerkner P, Herve M. emmeans: Estimated Marginal Means, aka Least-Squares Means (Version R package version 1.7.2), 2022. Available from: https://CRAN.R-project.org/package=emmeans
Signorell A. DescTools: Tools for descriptive statistics (Version R package version 0.99.44), February 3, 2024. Available from: https://CRAN.R-project.org/package=DescTools
Nakazawa M. fmsb: Functions for Medical Statistics Book with some Demographic Data. R package version 0.7.0., 2019. Available from: https://CRAN.R-project.org/package=fmsb
Wickham H. ggplot2: Elegant Graphics for Data Analysis. 2nd ed. Springer Cham; 2016. https://doi.org/10.1007/978-3-319-24277-4
R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2020. Available from: https://www.R-project.org/
Bonifacino M. A new species for the bee fauna of Italy: Dasypoda crassicornis (FRIESE, 1896) (Hymenoptera: Apoidea: Melittidae: Dasypodainae). Osmia. 2021;9:1–6.
Oertli S, Müller A, Dorn S. Ecological and seasonal patterns in the diversity of a species-rich bee assemblage (Hymenoptera: Apoidea: Apiformes). Eur J Entomol. 2005;102(1):53-63. doi:10.14411/eje.2005.008
Izsák J. Parameter dependence of correlation between the Shannon index and members of parametric diversity index family. Ecol Indic. 2007;7(1):181-194. doi:10.1016/j.ecolind.2005.12.001
Lu H-P, Wagner HH, Chen X-Y. A contribution diversity approach to evaluate species diversity. Basic Appl Ecol. 2007;8(1):1-12. doi:10.1016/j.baae.2006.06.004.
Hegland SJ, Nielsen A, Lázaro A, Bjerknes AL, Totland Ø. How does climate warming affect plant-pollinator interactions? Ecol Lett. 2009 Feb;12(2):184-95. doi: 10.1111/j.1461-0248.2008.01269.x. Epub 2008 Nov 26. PMID: 19049509.
Reddy P, Verghese A, Rajan VV. Potential impact of climate change on honeybees (Apis spp.) and their pollination services. Pest Manag Horticult Ecosyst. 2012;18(2):121-127.
Papanikolaou AD, Kühn I, Frenzel M, Schweiger O, Kleijn D. Semi‐natural habitats mitigate the effects of temperature rise on wild bees. J Appl Ecol. 2017;54(2):527-536. doi:10.1111/1365-2664.12763
Moreno JM. Preliminary assessment of the impacts in Spain due to the effect of climate change. Bol CF+ S. 2014;(38/39).
Fonseca D, Carvalho M, Marta-Almeida M, Melo-Gonçalves P, Rocha A. Recent trends of extreme temperature indices for the Iberian Peninsula. Phys Chem Earth Parts A/B/C. 2016;94:66-76.
Viceto C, Cardoso Pereira S, Rocha A. Climate change projections of extreme temperatures for the Iberian Peninsula. Atmosphere. 2019;10(5):229.
Ramírez F, Davenport TL. Apple pollination: A review. Scientia Horticulturae. 2013;162:188-203.
Gardner K, Ascher J. Notes on the native bee pollinators in New York apple orchards. J NY Entomol Soc. 2006;114(1):86-91.
Ornosa C, Torres F, Rúa P. Updated list of bumblebees (Hymenoptera: Apidae) from the Spanish Pyrenees with notes on their decline and conservation status. Zootaxa. 2017 Feb 26;4237(1):zootaxa.4237.1.3. doi: 10.11646/zootaxa.4237.1.3. PMID: 28264302.
Potts SG, Vulliamy B, Dafni A, Ne'eman G, Willmer P. Linking bees and flowers: how do floral communities structure pollinator communities? Ecology. 2003;84(10):2628-2642.
Bosch J, González AM, Rodrigo A, Navarro D. Plant-pollinator networks: adding the pollinator's perspective. Ecol Lett. 2009 May;12(5):409-19. doi: 10.1111/j.1461-0248.2009.01296.x. PMID: 19379135.
Flo V, Bosch J, Arnan X, Primante C, Martín González AM, Barril-Graells H, Rodrigo A. Yearly fluctuations of flower landscape in a Mediterranean scrubland: Consequences for floral resource availability. PLoS One. 2018 Jan 18;13(1):e0191268. doi: 10.1371/journal.pone.0191268. PMID: 29346453; PMCID: PMC5773194.
Stenseth NC, Mysterud A. Climate, changing phenology, and other life history traits: nonlinearity and match-mismatch to the environment. Proc Natl Acad Sci U S A. 2002 Oct 15;99(21):13379-81. doi: 10.1073/pnas.212519399. Epub 2002 Oct 7. PMID: 12370424; PMCID: PMC129680.
Visser ME, Both C. Shifts in phenology due to global climate change: the need for a yardstick. Proc Biol Sci. 2005 Dec 22;272(1581):2561-9. doi: 10.1098/rspb.2005.3356. PMID: 16321776; PMCID: PMC1559974.
Chole H, Woodard SH, Bloch G. Body size variation in bees: regulation, mechanisms, and relationship to social organization. Curr Opin Insect Sci. 2019 Oct;35:77-87. doi: 10.1016/j.cois.2019.07.006. Epub 2019 Jul 19. PMID: 31426016.
Mallinger RE, Gaines-Day HR, Gratton C. Do managed bees have negative effects on wild bees?: A systematic review of the literature. PLoS One. 2017 Dec 8;12(12):e0189268. doi: 10.1371/journal.pone.0189268. PMID: 29220412; PMCID: PMC5722319.
Angelella GM, McCullough CT, O'Rourke ME. Honey bee hives decrease wild bee abundance, species richness, and fruit count on farms regardless of wildflower strips. Sci Rep. 2021 Feb 5;11(1):3202. doi: 10.1038/s41598-021-81967-1. Erratum in: Sci Rep. 2021 Aug 17;11(1):17043. doi: 10.1038/s41598-021-95368-x. PMID: 33547371; PMCID: PMC7865060.
Weekers T, Marshall L, Leclercq N, Wood TJ, Cejas D, Drepper B, et al. Dominance of honey bees is negatively associated with wild bee diversity in commercial apple orchards regardless of management practices. Agric Ecosyst Environ. 2022;323:107697.
Iwasaki JM, Hogendoorn K. Mounting evidence that managed and introduced bees have negative impacts on wild bees: an updated review. Curr Res Insect Sci. 2022 Jul 22;2:100043. doi: 10.1016/j.cris.2022.100043. PMID: 36003276; PMCID: PMC9387436.
MacInnis G, Normandin E, Ziter CD. Decline in wild bee species richness associated with honey bee (Apis mellifera) abundance in an urban ecosystem. PeerJ. 2023 Feb 3;11:e14699. doi: 10.7717/peerj.14699. PMID: 36755869; PMCID: PMC9901307.
Paini D. Impact of the introduced honey bee (Apis mellifera) (Hymenoptera: Apidae) on native bees: a review. Austral Ecol. 2004;29(4):399-407.
Valido A, Rodríguez-Rodríguez MC, Jordano P. Honeybees disrupt the structure and functionality of plant-pollinator networks. Sci Rep. 2019 Mar 18;9(1):4711. doi: 10.1038/s41598-019-41271-5. PMID: 30886227; PMCID: PMC6423295.
Corbet SA, Williams IH, Osborne JL. Bees and the pollination of crops and wild flowers in the European Community. Bee World. 1991;72(2):47-59.
Rader R, Cunningham SA, Howlett BG, Inouye DW. Non-Bee Insects as Visitors and Pollinators of Crops: Biology, Ecology, and Management. Annu Rev Entomol. 2020 Jan 7;65:391-407. doi: 10.1146/annurev-ento-011019-025055. Epub 2019 Oct 14. PMID: 31610136.
García-González F, Ornosa C. Composition and identity of pollinators of blackberry (Rubus ulmifolius Schott, 1818) in Central Spain. Baetica Zoo. 1998;9:69-90.
Romero D, Ornosa C, Vargas P, Olesen JM. Solitary bees (Hymenoptera, Apoidea) as connectors in pollination networks: the case of Rhodanthidium. Apidologie. 2020;51(5):844-854.
Romero D, Vargas P, Ornosa C. Some traits of the biology of the red snail bee, Rhodanthidium sticticum (Fabricius, 1787): phenology, floral preference, shell use, flight capacity and territorial behavior. Graellsia. 2021;77(2):146.
Barbir J, Badenes-Pérez FR, Fernández-Quintanilla C, Dorado J. The attractiveness of flowering herbaceous plants to bees (Hymenoptera: Apoidea) and hoverflies (Diptera: Syrphidae) in agro-ecosystems of Central Spain. Agric Forest Entomol. 2015;17(1):20-28.
Teixido AL, Méndez M, Valladares F. Flower size and longevity influence florivory in the large-flowered shrub Cistus ladanifer. Acta Oecol. 2011;37(5):418-421.
Rader R, Bartomeus I, Garibaldi LA, Garratt MP, Howlett BG, Winfree R, Cunningham SA, Mayfield MM, Arthur AD, Andersson GK, Bommarco R, Brittain C, Carvalheiro LG, Chacoff NP, Entling MH, Foully B, Freitas BM, Gemmill-Herren B, Ghazoul J, Griffin SR, Gross CL, Herbertsson L, Herzog F, Hipólito J, Jaggar S, Jauker F, Klein AM, Kleijn D, Krishnan S, Lemos CQ, Lindström SA, Mandelik Y, Monteiro VM, Nelson W, Nilsson L, Pattemore DE, Pereira Nde O, Pisanty G, Potts SG, Reemer M, Rundlöf M, Sheffield CS, Scheper J, Schüepp C, Smith HG, Stanley DA, Stout JC, Szentgyörgyi H, Taki H, Vergara CH, Viana BF, Woyciechowski M. Non-bee insects are important contributors to global crop pollination. Proc Natl Acad Sci U S A. 2016 Jan 5;113(1):146-51. doi: 10.1073/pnas.1517092112. Epub 2015 Nov 30. PMID: 26621730; PMCID: PMC4711867.
Vargas P, Liberal I, Ornosa C, Gómez JM. Flower specialisation: the occluded corolla of snapdragons (Antirrhinum) exhibits two pollinator niches of large long-tongued bees. Plant Biol (Stuttg). 2017 Sep;19(5):787-797. doi: 10.1111/plb.12588. Epub 2017 Jul 7. PMID: 28590517.
Földesi R, Howlett BG, Grass I, Batáry P, Magrach A. Larger pollinators deposit more pollen on stigmas across multiple plant species—A meta‐ J Appl Ecol. 2020;58(4):699-707. doi:10.1111/1365-2664.13798
Guyot-Declerck C, Renson S, Bouseta A, Collin S. Floral quality and discrimination of Lavandula stoechas, Lavandula angustifolia, and Lavandula angustifolia × latifolia honeys. Food Chem. 2002;79(4):453-459.
Higginson A, Gilbert F, Barnard C. Morphological correlates of nectar production used by honeybees. Ecol Entomol. 2006;31(3):269-276.
Balfour NJ, Garbuzov M, Ratnieks FL. Longer tongues and swifter handling: why do more bumble bees (Bombus spp.) than honey bees (Apis mellifera) forage on lavender (Lavandula spp.)? Ecol Entomol. 2013;38(4):323-329.
Barrio M, Teixido AL. Sex-dependent selection on flower size in a large-flowered Mediterranean species: an experimental approach with Cistus ladanifer. Plant Syst Evol. 2015;301(1):113-124.
Teixido AL, Barrio M, Valladares F. Size matters: understanding the conflict faced by large flowers in Mediterranean environments. Bot Rev. 2016;82(2):204-228.
McKerchar M, Potts S, Fountain M, Garratt MP, Westbury DB. The potential for wildflower interventions to enhance natural enemies and pollinators in commercial apple orchards is limited by other management practices. Agric Ecosyst Environ. 2020;301:107034.
García RR, Miñarro M. Role of floral resources in the conservation of pollinator communities in cider-apple orchards. Agric Ecosyst Environ. 2014;183:118-126.
Maebe K, Hart AF, Marshall L, Vandamme P, Vereecken NJ, Michez D, Smagghe G. Bumblebee resilience to climate change, through plastic and adaptive responses. Glob Chang Biol. 2021 Sep;27(18):4223-4237. doi: 10.1111/gcb.15751. Epub 2021 Jun 30. PMID: 34118096.
Rohde AT, Branstetter MG, Mock KE, Knoblett JN, Pilliod DS, Everett JG, et al. Population genetics of museum specimens indicate decreasing genetic resiliency: The case of two bumble bees of conservation concern. Biol Conserv. 2024;291:110453.
Castroviejo S. Floristic Catalog. In: Jardín R, Botánico C, editors. Iberian Flora. Madrid: 1986-2012; 1-8:10-15; 17-18, 21.
Flora I. Iberian Flora: vascular plants of the Iberian Peninsula and Balearic Islands. http://www.floraiberica.es. Published 2020. Accessed March 26, 2024.
Gamonal N, Ornosa C. Assessing Bee (Hymenoptera, Apoidea, Anthophila) Diversity and Floral Preference in Two Habitats in the Iberian Peninsula. IgMin Res. Jul 02, 2024; 2(7): 490-502. IgMin ID: igmin208; DOI:10.61927/igmin208; Available at: igmin.link/p208
次のリンクを共有した人は、このコンテンツを読むことができます:
Address Correspondence:
Nerea Gamonal, Department of Biodiversity, Ecology and Evolution, Faculty of Biological Sciences, Complutense University, 28040, Madrid, Spain, Email: [email protected]
How to cite this article:
Gamonal N, Ornosa C. Assessing Bee (Hymenoptera, Apoidea, Anthophila) Diversity and Floral Preference in Two Habitats in the Iberian Peninsula. IgMin Res. Jul 02, 2024; 2(7): 490-502. IgMin ID: igmin208; DOI:10.61927/igmin208; Available at: igmin.link/p208
Copyright: © 2024 Gamonal N, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
 Figure 1: Location of the study sites, the thicket and the g...
Figure 1: Location of the study sites, the thicket and the g...
 Figure 2: Number of bee individuals collected on each month ...
Figure 2: Number of bee individuals collected on each month ...
 Figure 3: Number of bee individuals collected on each plant ...
Figure 3: Number of bee individuals collected on each plant ...
![<p>Bee composition was found across the grassland and the thicket in Galapagar from 2017 to 2019. Classification based on Ortiz-Sánchez [22].</p>](https://www.igminresearch.jp/articles/fulltext/table_images/igmin208/Table1.png) Table 1: Bee composition was found across the grassland and...
Table 1: Bee composition was found across the grassland and...
Fründ J, Linsenmair KE, Blüthgen N. Pollinator diversity and specialization in relation to flower diversity. Oikos. 2010;119(10):1581-1590. doi:10.1111/j.1600-0706.2010.18450.x
Nieto A, Roberts S, Kemp J, Rasmont P, Kuhlmann M, García Criado M, et al. European Red List of bees. Luxembourg: Publication Office of the European Union; 2014. 84 p.
Food and Agriculture Organization (FAO). Es hora de apreciar la labor de los polinizadores. https://www.fao.org/fao-stories/article/es/c/1129811/. Published 2018. Accessed March 25, 2024.
Díaz SM, Settele J, Brondízio E, Ngo H, Guèze M, Agard J, et al. The global assessment report on biodiversity and ecosystem services: Summary for policy makers. 2019.
Cardoso P, Barton PS, Birkhofer K, Chichorro F, Deacon C, Fartmann T, et al. Scientists' warning to humanity on insect extinctions. Biol Conserv. 2020;242. doi:10.1016/j.biocon.2020.108426
Ortiz-Sánchez FJ, Aguado Martín LÓ, Ornosa Gallego C. Bee diversity in Spain. Population trend and conservation measures (Hymenoptera, Apoidea, Anthophila). Ecosistemas. 2018;27(2):3-8. doi:10.7818/ecos.1315
Smith Pardo AH. Las abejas de Porce familia colletidae (hymenoptera: apoidea) notas y claves para los géneros presentes en la zona de influencia del embalse Porce ii. Rev Fac Nac Agron Medellín. 1999.
Klein A-M. Plant-pollinator interactions in changing environment. Basic Appl Ecol. 2011;12(4):279-341.
Ollerton J, Winfree R, Tarrant S. How many flowering plants are pollinated by animals? Oikos. 2011;120(3):321-326. doi:10.1111/j.1600-0706.2010.18644.x
Pardo A, Borges PA. Worldwide importance of insect pollination in apple orchards: A review. Agric Ecosyst Environ. 2020;293:106839.
Müller A, Diener S, Schnyder S, Stutz K, Sedivy C, Dorn S. Quantitative pollen requirements of solitary bees: implications for bee conservation and the evolution of bee–flower relationships. Biol Conserv. 2006;130(4):604-615.
Michener CD. The Bees of the World. 2nd ed. Baltimore: John Hopkins University Press; 2007.
Fründ J, Dormann CF, Holzschuh A, Tscharntke T. Bee diversity effects on pollination depend on functional complementarity and niche shifts. Ecology. 2013 Sep;94(9):2042-54. doi: 10.1890/12-1620.1. PMID: 24279275.
Leonhardt SD, Gallai N, Garibaldi LA, Kuhlmann M, Klein A-M. Economic gain, stability of pollination and bee diversity decrease from southern to northern Europe. Basic Appl Ecol. 2013;14(6):461-471.
Martínez-Puc JF, Merlo-Maydana FE. Importance of diversity of bees (Hymenoptera: Apoidea) and threats facing the tropical ecosystem of Yucatan, Mexico. J Selva Andina Anim Sci. 2014;1(2):28-34.
Khalifa SAM, Elshafiey EH, Shetaia AA, El-Wahed AAA, Algethami AF, Musharraf SG, AlAjmi MF, Zhao C, Masry SHD, Abdel-Daim MM, Halabi MF, Kai G, Al Naggar Y, Bishr M, Diab MAM, El-Seedi HR. Overview of Bee Pollination and Its Economic Value for Crop Production. Insects. 2021 Jul 31;12(8):688. doi: 10.3390/insects12080688. PMID: 34442255; PMCID: PMC8396518.
Quinet M, Warzée M, Vanderplanck M, Michez D, Lognay G, Jacquemart A-L. Do floral resources influence pollination rates and subsequent fruit set in pear (Pyrus communis L.) and apple (Malus x domestica Borkh) cultivars? Eur J Agron. 2016;77:59-69.
Reverté S, Bosch J, Arnan X, Roslin T, Stefanescu C, Calleja JA, et al. Spatial variability in a plant–pollinator community across a continuous habitat: high heterogeneity in the face of apparent uniformity. Ecography. 2019;42(9):1558-1568. doi:10.1111/ecog.04498.
Nunes-Silva P, Witter S, da Rosa JM, Halinski R, Schlemmer LM, Arioli CJ, Ramos JD, Botton M, Blochtein B. Diversity of Floral Visitors in Apple Orchards: Influence on Fruit Characteristics Depends on Apple Cultivar. Neotrop Entomol. 2020 Aug;49(4):511-524. doi: 10.1007/s13744-020-00762-1. Epub 2020 Mar 11. PMID: 32162245.
Ortiz-Sánchez FJ. Updated list of bee species in Spain (Hymenoptera: Apoidea: Apiformes). SEA Bowl. 2011;(49):265-281.
Álvarez Fidalgo P, Álvarez Fidalgo M, Noval Fonseca N, Castro L. Faunistic data on bees from the provinces of Asturias and León (northwest Spain), with a species not yet recorded in the Iberian Peninsula (Hymenoptera, Apoidea, Anthophila). Bol Asoc Esp Entomol. 2020;44.
Ortiz-Sánchez FJ. Iberian Fauna Checklist. Anthophila series (Insecta: Hymenoptera: Apoidea) in the Iberian Peninsula and Balearic Islands, 2020 ed. In: Ramos MA, Sánchez Ruiz M, eds. Iberian Fauna Documents. Madrid: National Museum of Natural Sciences, CSI; 2020. Vol. 14, pp. 83.
Ortiz-Sánchez F, del Castillo CR, Nieves-Aldrey J. Abundancia, Diversity and seasonal variation of apoidean genera (Hymenoptera, Apoidea) in two natural enclaves of the Community of Madrid (central Spain). Soc Entomol Aragonese Bowl. 2006;1(38):247-259.
Falk S. Field guide to the bees of Great Britain and Ireland. London: Bloomsbury Publishing; 2019.
Ornosa C, Ortiz-Sánchez FJ. Hymenoptera, Apoidea I. In: Ramos MA, et al., eds. Fauna Iberica. Madrid: Museo Nacional de Ciencias Naturales, CSIC; 2004. Vol. 23, pp. 556.
Dardón MJ, Torres F, Ornosa C. The subgenus Andrena (Micrandrena) (Hymenoptera: Andrenidae) in the Iberian Peninsula. Zootaxa. 2014 Oct 13;3872(5):467-97. doi: 10.11646/zootaxa.3872.5.3. PMID: 25544097.
Ortiz-Sánchez F, Ornosa C, Kuhlmann M. Identification keys for the Iberian species of the genus Colletes Latreille, 1802 (Hymenoptera, Colletidae). Zool Baetica. 2004;15:3-37.
Turley NE, Biddinger DJ, Joshi NK, López-Uribe MM. Six years of wild bee monitoring shows changes in biodiversity within and across years and declines in abundance. Ecol Evol. 2022 Aug 12;12(8):e9190. doi: 10.1002/ece3.9190. PMID: 35983174; PMCID: PMC9374588.
Gérard M, Vanderplanck M, Wood T, Michez D. Global warming and plant-pollinator mismatches. Emerg Top Life Sci. 2020 Jul 2;4(1):77-86. doi: 10.1042/ETLS20190139. PMID: 32558904; PMCID: PMC7326340.
Zattara EE, Aizen MA. Worldwide occurrence records suggest a global decline in bee species richness. One Earth. 2021;4(1):114-123. doi:10.1016/j.oneear.2020.12.005
Goulson D. Pesticides, Corporate Irresponsibility, and the Fate of Our Planet. One Earth. 2020;2(4):302-305. doi:10.1016/j.oneear.2020.03.004
Scheper J, Reemer M, van Kats R, Ozinga WA, van der Linden GT, Schaminée JH, Siepel H, Kleijn D. Museum specimens reveal loss of pollen host plants as key factor driving wild bee decline in The Netherlands. Proc Natl Acad Sci U S A. 2014 Dec 9;111(49):17552-7. doi: 10.1073/pnas.1412973111. Epub 2014 Nov 24. PMID: 25422416; PMCID: PMC4267333.
Klein AM, Vaissière BE, Cane JH, Steffan-Dewenter I, Cunningham SA, Kremen C, Tscharntke T. Importance of pollinators in changing landscapes for world crops. Proc Biol Sci. 2007 Feb 7;274(1608):303-13. doi: 10.1098/rspb.2006.3721. PMID: 17164193; PMCID: PMC1702377.
Joshi NK, Otieno M, Rajotte EG, Fleischer SJ, Biddinger DJ. Proximity to woodland and landscape structure drives pollinator visitation in apple orchard ecosystem. Front Ecol Evol. 2016;4:38.
Colla SR. The potential consequences of 'bee washing' on wild bee health and conservation. Int J Parasitol Parasites Wildl. 2022 Apr 2;18:30-32. doi: 10.1016/j.ijppaw.2022.03.011. PMID: 35399591; PMCID: PMC8989764.
Zurbuchen A, Landert L, Klaiber J, Müller A, Hein S, Dorn S. Maximum Foraging Ranges in Solitary Bees: Only Few Individuals have the Capability to Cover Long Foraging Distances. Biol Conserv. 2010;143:669-676. doi:10.1016/j.biocon.2009.12.003.
Kendall LK, Mola JM, Portman ZM, Cariveau DP, Smith HG, Bartomeus I. The potential and realized foraging movements of bees are differentially determined by body size and sociality. Ecology. 2022 Nov;103(11):e3809. doi: 10.1002/ecy.3809. Epub 2022 Sep 1. PMID: 35792515; PMCID: PMC9786665.
Roswell M, Dushoff J, Winfree R. A conceptual guide to measuring species diversity. Oikos. 2021;130(3):321-338. doi:10.1111/oik.07202
Chao A, Gotelli NJ, Hsieh T, Sander EL, Ma K, Colwell RK, et al. Rarefaction and extrapolation with Hill numbers: a framework for sampling and estimation in species diversity studies. Ecol Monogr. 2014;84(1):45-67.
Gotelli NJ, Chao A. Measuring and estimating species richness, species diversity, and biotic similarity from sampling data. In: Levin SA, ed. Encyclopedia of Biodiversity. 2nd ed. Vol. 5. Oxford: Academic Press; 2013; 195-211.
Russo L, Park M, Gibbs J, Danforth B. The challenge of accurately documenting bee species richness in agroecosystems: bee diversity in eastern apple orchards. Ecol Evol. 2015 Sep;5(17):3531-40. doi: 10.1002/ece3.1582. Epub 2015 Aug 5. PMID: 26380684; PMCID: PMC4567859.
Hsieh T, Ma K, Chao A. iNEXT: an R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol Evol. 2016;7(12):1451-1456.
Chafee M, Fernàndez-Guerra A, Buttigieg PL, Gerdts G, Eren AM, Teeling H, Amann RI. Recurrent patterns of microdiversity in a temperate coastal marine environment. ISME J. 2018 Jan;12(1):237-252. doi: 10.1038/ismej.2017.165. Epub 2017 Oct 24. PMID: 29064479; PMCID: PMC5739018.
Chao A, Kubota Y, Zelený D, Chiu CH, Li CF, Kusumoto B, et al. Quantifying sample completeness and comparing diversities among assemblages. Ecol Res. 2020;35(2):292-314.
Magurran AE. Diversidad ecológica y su medición. Barcelona: Ediciones Vedra; 1989.
Higes M, Martín R, Meana A. Nosema ceranae, a new microsporidian parasite in honeybees in Europe. J Invertebr Pathol. 2006 Jun;92(2):93-5. doi: 10.1016/j.jip.2006.02.005. Epub 2006 Mar 29. PMID: 16574143.
Yeom DJ, Kim JH. Comparative evaluation of species diversity indices in the natural deciduous forest of Mt. Jeombong. For Sci Technol. 2011;7(2):68-74. doi:10.1080/21580103.2011.573940.
Supriatna J. Biodiversity Indexes: Value and evaluation purposes. Paper presented at: E3S Web of Conferences; 2018. doi:10.1051/e3sconf/20184801001
Nisa RU, Gupta K, Wani SM, Allie KA, Kouser N. A study on diversity and ecology of ichthyofauna of Rajouri district, Jammu and Kashmir, India. Rec Zool Surv India. 2021;120(4):363-372.
Oksanen J, Blanchet FG, Guillaume Guénard, Friendly M, Kindt R, Legendre P, et al. vegan: Community Ecology Package (Version R package version 2.5-7), November 2020. Available from: https://CRAN.R-project.org/package=vegan
Lenth RV, Singmann H, Love J, Buerkner P, Herve M. emmeans: Estimated Marginal Means, aka Least-Squares Means (Version R package version 1.7.2), 2022. Available from: https://CRAN.R-project.org/package=emmeans
Signorell A. DescTools: Tools for descriptive statistics (Version R package version 0.99.44), February 3, 2024. Available from: https://CRAN.R-project.org/package=DescTools
Nakazawa M. fmsb: Functions for Medical Statistics Book with some Demographic Data. R package version 0.7.0., 2019. Available from: https://CRAN.R-project.org/package=fmsb
Wickham H. ggplot2: Elegant Graphics for Data Analysis. 2nd ed. Springer Cham; 2016. https://doi.org/10.1007/978-3-319-24277-4
R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2020. Available from: https://www.R-project.org/
Bonifacino M. A new species for the bee fauna of Italy: Dasypoda crassicornis (FRIESE, 1896) (Hymenoptera: Apoidea: Melittidae: Dasypodainae). Osmia. 2021;9:1–6.
Oertli S, Müller A, Dorn S. Ecological and seasonal patterns in the diversity of a species-rich bee assemblage (Hymenoptera: Apoidea: Apiformes). Eur J Entomol. 2005;102(1):53-63. doi:10.14411/eje.2005.008
Izsák J. Parameter dependence of correlation between the Shannon index and members of parametric diversity index family. Ecol Indic. 2007;7(1):181-194. doi:10.1016/j.ecolind.2005.12.001
Lu H-P, Wagner HH, Chen X-Y. A contribution diversity approach to evaluate species diversity. Basic Appl Ecol. 2007;8(1):1-12. doi:10.1016/j.baae.2006.06.004.
Hegland SJ, Nielsen A, Lázaro A, Bjerknes AL, Totland Ø. How does climate warming affect plant-pollinator interactions? Ecol Lett. 2009 Feb;12(2):184-95. doi: 10.1111/j.1461-0248.2008.01269.x. Epub 2008 Nov 26. PMID: 19049509.
Reddy P, Verghese A, Rajan VV. Potential impact of climate change on honeybees (Apis spp.) and their pollination services. Pest Manag Horticult Ecosyst. 2012;18(2):121-127.
Papanikolaou AD, Kühn I, Frenzel M, Schweiger O, Kleijn D. Semi‐natural habitats mitigate the effects of temperature rise on wild bees. J Appl Ecol. 2017;54(2):527-536. doi:10.1111/1365-2664.12763
Moreno JM. Preliminary assessment of the impacts in Spain due to the effect of climate change. Bol CF+ S. 2014;(38/39).
Fonseca D, Carvalho M, Marta-Almeida M, Melo-Gonçalves P, Rocha A. Recent trends of extreme temperature indices for the Iberian Peninsula. Phys Chem Earth Parts A/B/C. 2016;94:66-76.
Viceto C, Cardoso Pereira S, Rocha A. Climate change projections of extreme temperatures for the Iberian Peninsula. Atmosphere. 2019;10(5):229.
Ramírez F, Davenport TL. Apple pollination: A review. Scientia Horticulturae. 2013;162:188-203.
Gardner K, Ascher J. Notes on the native bee pollinators in New York apple orchards. J NY Entomol Soc. 2006;114(1):86-91.
Ornosa C, Torres F, Rúa P. Updated list of bumblebees (Hymenoptera: Apidae) from the Spanish Pyrenees with notes on their decline and conservation status. Zootaxa. 2017 Feb 26;4237(1):zootaxa.4237.1.3. doi: 10.11646/zootaxa.4237.1.3. PMID: 28264302.
Potts SG, Vulliamy B, Dafni A, Ne'eman G, Willmer P. Linking bees and flowers: how do floral communities structure pollinator communities? Ecology. 2003;84(10):2628-2642.
Bosch J, González AM, Rodrigo A, Navarro D. Plant-pollinator networks: adding the pollinator's perspective. Ecol Lett. 2009 May;12(5):409-19. doi: 10.1111/j.1461-0248.2009.01296.x. PMID: 19379135.
Flo V, Bosch J, Arnan X, Primante C, Martín González AM, Barril-Graells H, Rodrigo A. Yearly fluctuations of flower landscape in a Mediterranean scrubland: Consequences for floral resource availability. PLoS One. 2018 Jan 18;13(1):e0191268. doi: 10.1371/journal.pone.0191268. PMID: 29346453; PMCID: PMC5773194.
Stenseth NC, Mysterud A. Climate, changing phenology, and other life history traits: nonlinearity and match-mismatch to the environment. Proc Natl Acad Sci U S A. 2002 Oct 15;99(21):13379-81. doi: 10.1073/pnas.212519399. Epub 2002 Oct 7. PMID: 12370424; PMCID: PMC129680.
Visser ME, Both C. Shifts in phenology due to global climate change: the need for a yardstick. Proc Biol Sci. 2005 Dec 22;272(1581):2561-9. doi: 10.1098/rspb.2005.3356. PMID: 16321776; PMCID: PMC1559974.
Chole H, Woodard SH, Bloch G. Body size variation in bees: regulation, mechanisms, and relationship to social organization. Curr Opin Insect Sci. 2019 Oct;35:77-87. doi: 10.1016/j.cois.2019.07.006. Epub 2019 Jul 19. PMID: 31426016.
Mallinger RE, Gaines-Day HR, Gratton C. Do managed bees have negative effects on wild bees?: A systematic review of the literature. PLoS One. 2017 Dec 8;12(12):e0189268. doi: 10.1371/journal.pone.0189268. PMID: 29220412; PMCID: PMC5722319.
Angelella GM, McCullough CT, O'Rourke ME. Honey bee hives decrease wild bee abundance, species richness, and fruit count on farms regardless of wildflower strips. Sci Rep. 2021 Feb 5;11(1):3202. doi: 10.1038/s41598-021-81967-1. Erratum in: Sci Rep. 2021 Aug 17;11(1):17043. doi: 10.1038/s41598-021-95368-x. PMID: 33547371; PMCID: PMC7865060.
Weekers T, Marshall L, Leclercq N, Wood TJ, Cejas D, Drepper B, et al. Dominance of honey bees is negatively associated with wild bee diversity in commercial apple orchards regardless of management practices. Agric Ecosyst Environ. 2022;323:107697.
Iwasaki JM, Hogendoorn K. Mounting evidence that managed and introduced bees have negative impacts on wild bees: an updated review. Curr Res Insect Sci. 2022 Jul 22;2:100043. doi: 10.1016/j.cris.2022.100043. PMID: 36003276; PMCID: PMC9387436.
MacInnis G, Normandin E, Ziter CD. Decline in wild bee species richness associated with honey bee (Apis mellifera) abundance in an urban ecosystem. PeerJ. 2023 Feb 3;11:e14699. doi: 10.7717/peerj.14699. PMID: 36755869; PMCID: PMC9901307.
Paini D. Impact of the introduced honey bee (Apis mellifera) (Hymenoptera: Apidae) on native bees: a review. Austral Ecol. 2004;29(4):399-407.
Valido A, Rodríguez-Rodríguez MC, Jordano P. Honeybees disrupt the structure and functionality of plant-pollinator networks. Sci Rep. 2019 Mar 18;9(1):4711. doi: 10.1038/s41598-019-41271-5. PMID: 30886227; PMCID: PMC6423295.
Corbet SA, Williams IH, Osborne JL. Bees and the pollination of crops and wild flowers in the European Community. Bee World. 1991;72(2):47-59.
Rader R, Cunningham SA, Howlett BG, Inouye DW. Non-Bee Insects as Visitors and Pollinators of Crops: Biology, Ecology, and Management. Annu Rev Entomol. 2020 Jan 7;65:391-407. doi: 10.1146/annurev-ento-011019-025055. Epub 2019 Oct 14. PMID: 31610136.
García-González F, Ornosa C. Composition and identity of pollinators of blackberry (Rubus ulmifolius Schott, 1818) in Central Spain. Baetica Zoo. 1998;9:69-90.
Romero D, Ornosa C, Vargas P, Olesen JM. Solitary bees (Hymenoptera, Apoidea) as connectors in pollination networks: the case of Rhodanthidium. Apidologie. 2020;51(5):844-854.
Romero D, Vargas P, Ornosa C. Some traits of the biology of the red snail bee, Rhodanthidium sticticum (Fabricius, 1787): phenology, floral preference, shell use, flight capacity and territorial behavior. Graellsia. 2021;77(2):146.
Barbir J, Badenes-Pérez FR, Fernández-Quintanilla C, Dorado J. The attractiveness of flowering herbaceous plants to bees (Hymenoptera: Apoidea) and hoverflies (Diptera: Syrphidae) in agro-ecosystems of Central Spain. Agric Forest Entomol. 2015;17(1):20-28.
Teixido AL, Méndez M, Valladares F. Flower size and longevity influence florivory in the large-flowered shrub Cistus ladanifer. Acta Oecol. 2011;37(5):418-421.
Rader R, Bartomeus I, Garibaldi LA, Garratt MP, Howlett BG, Winfree R, Cunningham SA, Mayfield MM, Arthur AD, Andersson GK, Bommarco R, Brittain C, Carvalheiro LG, Chacoff NP, Entling MH, Foully B, Freitas BM, Gemmill-Herren B, Ghazoul J, Griffin SR, Gross CL, Herbertsson L, Herzog F, Hipólito J, Jaggar S, Jauker F, Klein AM, Kleijn D, Krishnan S, Lemos CQ, Lindström SA, Mandelik Y, Monteiro VM, Nelson W, Nilsson L, Pattemore DE, Pereira Nde O, Pisanty G, Potts SG, Reemer M, Rundlöf M, Sheffield CS, Scheper J, Schüepp C, Smith HG, Stanley DA, Stout JC, Szentgyörgyi H, Taki H, Vergara CH, Viana BF, Woyciechowski M. Non-bee insects are important contributors to global crop pollination. Proc Natl Acad Sci U S A. 2016 Jan 5;113(1):146-51. doi: 10.1073/pnas.1517092112. Epub 2015 Nov 30. PMID: 26621730; PMCID: PMC4711867.
Vargas P, Liberal I, Ornosa C, Gómez JM. Flower specialisation: the occluded corolla of snapdragons (Antirrhinum) exhibits two pollinator niches of large long-tongued bees. Plant Biol (Stuttg). 2017 Sep;19(5):787-797. doi: 10.1111/plb.12588. Epub 2017 Jul 7. PMID: 28590517.
Földesi R, Howlett BG, Grass I, Batáry P, Magrach A. Larger pollinators deposit more pollen on stigmas across multiple plant species—A meta‐ J Appl Ecol. 2020;58(4):699-707. doi:10.1111/1365-2664.13798
Guyot-Declerck C, Renson S, Bouseta A, Collin S. Floral quality and discrimination of Lavandula stoechas, Lavandula angustifolia, and Lavandula angustifolia × latifolia honeys. Food Chem. 2002;79(4):453-459.
Higginson A, Gilbert F, Barnard C. Morphological correlates of nectar production used by honeybees. Ecol Entomol. 2006;31(3):269-276.
Balfour NJ, Garbuzov M, Ratnieks FL. Longer tongues and swifter handling: why do more bumble bees (Bombus spp.) than honey bees (Apis mellifera) forage on lavender (Lavandula spp.)? Ecol Entomol. 2013;38(4):323-329.
Barrio M, Teixido AL. Sex-dependent selection on flower size in a large-flowered Mediterranean species: an experimental approach with Cistus ladanifer. Plant Syst Evol. 2015;301(1):113-124.
Teixido AL, Barrio M, Valladares F. Size matters: understanding the conflict faced by large flowers in Mediterranean environments. Bot Rev. 2016;82(2):204-228.
McKerchar M, Potts S, Fountain M, Garratt MP, Westbury DB. The potential for wildflower interventions to enhance natural enemies and pollinators in commercial apple orchards is limited by other management practices. Agric Ecosyst Environ. 2020;301:107034.
García RR, Miñarro M. Role of floral resources in the conservation of pollinator communities in cider-apple orchards. Agric Ecosyst Environ. 2014;183:118-126.
Maebe K, Hart AF, Marshall L, Vandamme P, Vereecken NJ, Michez D, Smagghe G. Bumblebee resilience to climate change, through plastic and adaptive responses. Glob Chang Biol. 2021 Sep;27(18):4223-4237. doi: 10.1111/gcb.15751. Epub 2021 Jun 30. PMID: 34118096.
Rohde AT, Branstetter MG, Mock KE, Knoblett JN, Pilliod DS, Everett JG, et al. Population genetics of museum specimens indicate decreasing genetic resiliency: The case of two bumble bees of conservation concern. Biol Conserv. 2024;291:110453.
Castroviejo S. Floristic Catalog. In: Jardín R, Botánico C, editors. Iberian Flora. Madrid: 1986-2012; 1-8:10-15; 17-18, 21.
Flora I. Iberian Flora: vascular plants of the Iberian Peninsula and Balearic Islands. http://www.floraiberica.es. Published 2020. Accessed March 26, 2024.