
Examining the Causal Connection between Lipid-lowering Medications and Malignant Meningiomas through Drug-target Mendelian Randomization Analysis
Pharmacology OncologyNeurology受け取った 11 Apr 2024 受け入れられた 14 May 2024 オンラインで公開された 15 May 2024
Focusing on Biology, Medicine and Engineering ISSN: 2995-8067 | Quick Google Scholar


受け取った 11 Apr 2024 受け入れられた 14 May 2024 オンラインで公開された 15 May 2024
Objectives: This study aims to investigate the causal link between the use of statins, a type of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitor, and the risk of developing malignant meningiomas, which are aggressive and recurrent tumors of the central nervous system with limited treatment options.
Methods: Using Mendelian Randomization (MR) analysis, the study explored the relationship between genetic variants related to the expression of lipid-lowering drug targets (HMGCR, PCSK9, NPC1L1, and APOB) and malignant meningiomas. The analysis utilized data from Genome-Wide Association Studies (GWAS) and expression quantitative trait loci (eQTL) databases, with a focus on the genetic homogeneity of the Finnish population. Instrumental variables for the MR analysis were derived from significant eQTLs for the mentioned drug targets.
Results: The MR analysis found a significant association between genetic variants linked to HMGCR inhibitor (statin) exposure and a reduced risk of malignant meningiomas. Specifically, an increased expression of the HMGCR gene in the blood was associated with lower susceptibility to malignant meningiomas (Odds Ratio [OR] = 2.57, 95% Confidence Interval [CI] = 1.05 - 6.31; p = 0.039). No significant associations were observed for other lipid-lowering drug targets.
Conclusion: Preliminary evidence suggests that statin use may lower the risk of developing malignant meningiomas, indicating a potential therapeutic benefit for managing this type of cancer. However, further research, including clinical trials, is necessary to confirm these findings and understand the mechanisms behind the protective effect of statins against malignant meningiomas.
Central nervous system tumors such as meningiomas are the most common []. The World Health Organization (WHO) categorizes meningiomas into Grades I, II, and III, with increasing grades associated with higher rates of occurrence and death. Meningiomas in grades II and III are distinguished by their unusual features and tendency to invade nearby tissues []. Approximately 80% of instances exhibit noncancerous histological features, which may be treated effectively with surgery, while the remaining 20% display cancerous histological attributes, increasing the risk of quick reappearance [,]. Adjunctive radiotherapy is frequently utilized to enhance local control, especially in malignant meningiomas. Yet, there is a void in effective pharmacological interventions. Meningiomas have not been successfully treated in several clinical trials [,]. Therefore, additional therapeutic strategies for malignant meningiomas are urgently needed.
As well as forming biological membranes, lipids serve as signaling molecules and energy sources. There are a number of biological processes that are influenced by cholesterol, its precursors, or metabolites, including cell immunity, post-translational modifications of proteins, and cell signaling [,]. It is also well documented that cancer cells’ perpetual growth is accompanied by an increased need for cholesterol, as many types of malignancies exhibit cholesterol-related metabolic problems [,]. Tumor growth and metastatic spread are caused by lipids that alter key processes such as the energy supply to tumor cells, the fluidity of cell membranes, and the signaling in tumor cells, offering a potential target for developing new treatments against metastatic cancer [].
Lipid homeostasis is currently regulated by various lipid-lowering medications based on lipid metabolism processes, but their targets are different. Common targets include 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase (HMGCR), PCSK9 proprotein convertase subtilisin/kexin family member (evolocumab and alirocumab), cholesterol transporter (NPC1L1, targeted by ezetimibe), and apolipoprotein B (ApoB, targeted by mipomersen). HMG-CoA reductase plays a crucial role as the enzyme that controls the speed of cholesterol and other isoprenoid production in the body. It speeds up the transformation of HMG-CoA into mevalonate, an essential stage in the production of cholesterol []. PCSK9, the ninth member of the proprotein convertase family, controls LDL cholesterol levels in the blood by interacting with the LDL receptor []. NPC1L1 plays a vital role in intestinal cholesterol absorption, promoting cholesterol uptake into absorptive cells of the small intestine through vesicular endocytosis []. ApoB is a component of several lipoproteins in the blood, including low-density and very low-density lipoproteins [].
Mendelian Randomization (MR) analysis is a new and strong epidemiological method that uses genetic variations as unbiased instrumental variables to study the causal connections between exposures and disease outcomes. Taking advantage of the fact that genotypes are established at conception and typically not influenced by confounding factors, MR analysis provides impartial estimates []. Additionally, MR can predict drug efficacy and reveal the potential utility of targets []. This analysis method has found widespread application in the pharmaceutical field. Wang, et al. employed this method to discover that novel hypoglycemic drugs could reduce the risk of cardiovascular diseases []. Ueda, et al. also used this approach to identify a link between targets for reducing uric acid and chronic kidney disease [].
This research examines the connection between cholesterol-lowering medications and cancerous brain tumors using genetic analysis, providing fresh perspectives on how to prevent and treat these tumors.
This study encompasses four types of lipid-lowering medications: inhibitors of HMGCR, PCSK9, NPC1L1, and APOB. An MR study comparing two samples was performed utilizing summary statistics from publicly accessible GWAS and eQTLs studies. Approval from appropriate institutional review boards was obtained for all studies, and participants provided informed consent.
The eQTLs of the drug target genes (HMGCR, PCSK9, NPC1L1, and APOB) were used as substitutes to represent exposure to various lipid-lowering medications. Data on eQTL summary statistics were collected from either the eQTLGen database (https://www.eQTLGen.org/) or the GTEx V8 database (https://gtexportal.org/) (Supplement Table 1). Significant associations (p<5.0×10^-8) were found between eQTLs with a minor allele frequency (MAF) > 1% and the expression of HMGCR and PCSK9 in blood, as well as NPC1L1 and APOB in subcutaneous adipose tissue. This was due to the lack of significant eQTLs for NPC1L1 and APOB in blood or other tissues. Genetic instruments were generated by including only cis-eQTLs located within a 1Mb distance from the coding gene.
Additionally, in order to confirm the connections identified using eQTLs as tools, a different method was utilized involving the selection of single nucleotide polymorphisms (SNPs) located within 100kb of each gene associated with LDL cholesterol levels, which reflects exposure to medications that lower lipids at a significant level across the entire genome (p < 5.0 × 10^-8). Variants were detected through analysis of data from the LDL cholesterol GWAS conducted by the Global Lipids Genetics Consortium (GLGC), involving 173,082 samples []. For HMGCR, 7 SNPs within a 100kb range were selected as instrumental variables, 12 for PCSK9, 3 for NPC1L1 inhibitors, and 20 for APOB. The threshold for linkage disequilibrium (R^2) of selected instrumental variable SNPs was set at 0.3 to maximize the strength of each drug’s instrumental variables.
Data from Genome-Wide Association Studies (GWAS) on malignant meningiomas and coronary artery atherosclerosis were acquired from the FinnGen research initiative at https://r5.finngen.fi/.FinnGen, a collaboration between the public and private sectors, merges genetic information from both new and preexisting samples from biobanks in Finland with electronic health record data from health registries in Finland, with the goal of uncovering fresh perspectives on the genetic basis of diseases []. The included malignant meningioma dataset comprises 174,646 total samples, including 640 cases of malignant meningiomas and 174,006 controls. The sample size for coronary artery atherosclerosis data was 218,792, including 23,363 cases of malignant meningiomas and 195,429 controls (Supplement Table 1).
The summary data-based MR (SMR) method was utilized with eQTLs as instruments to estimate effects, examining the association between gene expression levels and outcomes of interest by analyzing summary data from GWAS and eQTL studies []. The process of aligning alleles and conducting analyses was carried out with the use of SMR software, specifically version 1.03, which can be found at https://cnsgenomics.com/software/smr/#Overview. Using genetic variants associated with LDL cholesterol levels as instruments, the IVW method in the TwoSampleMR package in R software version 4.1.0 estimated the impact of lipid-lowering medications on results.
To assess the effectiveness of SNPs as instruments, F statistics were utilized, including SNPs with an F statistic greater than 10 to reduce the impact of weak instrument bias []. Furthermore, the instrumental variables were validated through positive control analyses. In the study of eQTLs, the connection between specific target genes and LDL cholesterol levels was examined as a positive control experiment for the instrumental variables due to the fact that reducing LDL cholesterol levels is a key effect of lipid-lowering drugs. For instrumental variables from the LDL cholesterol GWAS, a positive control study was conducted by examining the relationship between target genes and coronary artery atherosclerosis, given it is an indication for lipid-lowering medications.
The SMR method utilized the Heterogeneity in Dependent Instruments (HEIDI) to examine if the correlation between gene expression and outcomes was influenced by linkage disequilibrium, conducted within the SMR software []. A HEIDI test with a significance level of p < 0.01 suggests that the relationship could be attributed to genetic linkage []. A single SNP could impact the expression of several genes, resulting in horizontal pleiotropy. Identifying nearby genes (within a 1Mb range) whose expression was significantly linked to the genetic instrument variants was done to evaluate the risk of horizontal pleiotropy, followed by SMR analysis to investigate the potential relationship between the expression of these genes and the outcomes of malignant meningiomas.
Cochran’s Q test was utilized to evaluate heterogeneity in the IVW-MR method, with heterogeneity indicated by a
p - value < 0.05 []. MR-Egger regression and MR-PRESSO analysis were used to assess possible horizontal pleiotropy among the SNPs utilized as instrumental variables. The intercept in MR-Egger regression acts as a signal for directional pleiotropy, with a p - value < 0.05 indicating the existence of pleiotropy []. The MR-PRESSO analysis is capable of detecting pleiotropic anomalies, where a Global test p - value < 0.05 suggests the existence of pleiotropy and outliers []. All these analyses were implemented in R software version 4.1.0.
Genetic Instrument Selection and Outcomes with COVID-19.
From the eQTLGen or GTEx databases, a total of 921, 24, 11, and 161 cis-eQTLs were identified for the drug target genes HMGCR, PCSK9, NPC1L1, and APOB, respectively. The primary cis-eQTL SNP for each gene targeted by the drug was chosen as the genetic tool (Supplement Table 2). 7, 12, 3, and 20 SNPs were chosen for HMGCR, PCSK9, NPC1L1, and APOB from the summary data of the LDL-cholesterol level GWAS conducted by the Global Lipid Genetics Consortium (Supplement Table 3). The F statistics for all instrumental variables exceeded 20, indicating a low likelihood of bias from weak instruments in our analysis (Supplement Tables 2,3). Positive control experiments demonstrated notable connections between individual drug exposure and LDL cholesterol levels by utilizing eQTLs as instrumental variables (Supplement Table 5). Additionally, the association between drug exposure and coronary artery atherosclerosis was confirmed by LDL cholesterol GWAS, reinforcing the reliability of the chosen genetic tools (Supplement Table 6).
Findings from SMR analysis indicated a potential link between elevated levels of the HMGCR gene in the bloodstream (comparable to a one standard deviation increase) and vulnerability to malignant meningiomas (Odds Ratio [OR] = 2.70, 95% Confidence Interval [CI] = 1.07-6.81; p = 0.035), suggesting that inhibiting HMGCR could lower the chances of developing malignant meningiomas (Figure 1 and Supplement Table 2). There were no notable connections discovered between the levels of PCSK9, NPCIL1, APOB, and malignant meningiomas.
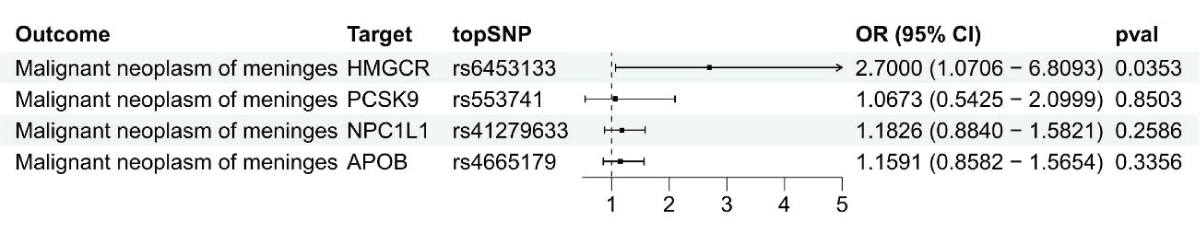 Figure 1: Using summary data, a Mendelian Randomization analysis was conducted to investigate the link between gene expression levels of HMGCR, PCSK9, NPC1L1, and APOB and malignant meningiomas.
Figure 1: Using summary data, a Mendelian Randomization analysis was conducted to investigate the link between gene expression levels of HMGCR, PCSK9, NPC1L1, and APOB and malignant meningiomas.IVW-MR analysis revealed a connection between LDL cholesterol controlled by HMGCR and the likelihood of malignant meningiomas (OR = 2.57, 95%CI = 1.05 - 6.31; p = 0.039), reinforcing the idea that HMGCR inhibitors could offer protection against malignant meningiomas (Figure 2 and Supplement Table 4). Strong evidence was found linking PCSK9-mediated LDL cholesterol with the risk of malignant meningiomas, with an odds ratio of 2.00 and a 95% confidence interval of 1.24 - 3.19, resulting in a p - value of 0.004. No evidence was provided by IVW-MR analysis for an association between NPC1L1 and APOB mediated LDL cholesterol and outcomes of malignant meningiomas.
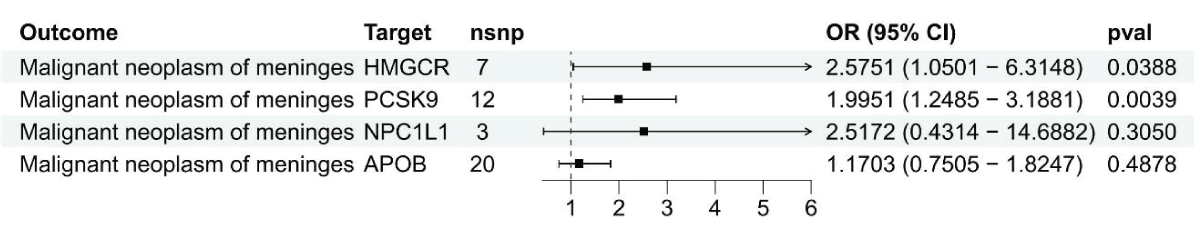 Figure 2: Results from the Inverse-Variance Weighted (IVW) method in Figure 2 show the connection between LDL cholesterol and malignant meningiomas through HMGCR, PCSK9, NPC1L1, and APOB genes.
Figure 2: Results from the Inverse-Variance Weighted (IVW) method in Figure 2 show the connection between LDL cholesterol and malignant meningiomas through HMGCR, PCSK9, NPC1L1, and APOB genes.For SMR analysis, HEIDI tests indicated that the observed outcomes were not due to inter-gene associations (p > 0.01). A more in-depth analysis was performed to investigate horizontal pleiotropy by examining the expression of neighboring genes linked to the top SNP associated with HMGCR and the prognosis of malignant meningiomas. Six genes, such as HMGCR, were found to have expression levels correlated with the instrumental variable (Supplement Table 7). Just three genes had eQTLs that reached genome-wide significance (p < 5.0 × 10^-8). Out of the three genes, only HMGCR expression showed a significant correlation with the outcomes of malignant meningiomas, indicating a limited impact of horizontal pleiotropy on the observed connections (Supplement Table 8). Cochran’s Q test for the IVW-MR analysis did not detect any heterogeneity among the reported results (p > 0.05; Supplement Table 4). The intercept values obtained from MR-Egger regression and MR-PRESSO analysis did not show statistical significance (p > 0.05; Supplement Table 4), suggesting a lack of pleiotropy in general.
Malignant meningiomas, characterized by their malignant tumor traits such as recurrent local recurrence and potential extracranial metastasis, present significant challenges in treatment. Caroline et al. recently reported that the frequency of primary malignant brain tumors is around 7 cases per 100,000 people []. Common therapies for malignant meningiomas consist of surgery to remove the tumor, radiosurgery after the operation, and the use of chemotherapy [-]. Surgical removal is the preferred and most effective method, yet postoperative peritumoral edema and severe brain edema can lead to life-threatening conditions such as brain herniation []. Postoperative radiation therapy is advocated by some, though not all patients benefit from it [,].
Due to the limited availability of reliable randomized controlled trial evidence backing the efficacy of lipid-lowering medications and the difficulties in carrying out extensive randomized controlled trials, Mendelian Randomization (MR) analysis provides a practical and precise epidemiological approach for studying the causal connections between exposure variables and results through the use of public databases. Our study provides evidence that inhibitors of HMGCR may reduce the risk of meningioma, offering a reference for patients using lipid-lowering medications.
This study investigated how the risk of meningiomas is affected by four different lipid-lowering drugs: HMGCR inhibitors, PCSK9 inhibitors, NPC1L1 inhibitors, and APOB inhibitors. We found potential evidence suggesting that HMGCR inhibitors could lower the risk of malignant meningiomas. No significant associations were observed for PCSK9, NPC1L1, and APOB expressions in relation to malignant meningiomas.SMR analysis findings indicated a potential link between elevated HMGCR expression in the bloodstream and vulnerability to malignant meningiomas, with an odds ratio of 2.70 and a 95% confidence interval of 1.07 - 6.81, suggesting a possible protective impact of HMGCR inhibitors. The IVW-MR analysis provided additional evidence for the potential benefit of statins in reducing the risk of malignant meningiomas (OR = 2.57, 95%CI = 1.05 - 6.31; p = 0.039).
Compared to the development of new pharmaceuticals, repurposing existing drugs offers a more economical and time-efficient approach. Due to the significant effects of statins in lowering Low-Density Lipoprotein Cholesterol, they are widely used in various diseases related to lipid metabolism, especially cardiovascular diseases [,]. Abundant evidence supports that the therapeutic advantages of statin treatment significantly surpass any real or perceived risks [,]. Due to their expiration of patent protections and consequent low overall costs, statins emerge as viable candidates for repurposing. Among the plethora of prescription drugs, statins draw considerable attention for their multifaceted benefits, including their ability to reduce serum cholesterol levels, their anti-inflammatory and immunomodulatory properties, and their antithrombotic actions. Together, these abilities could be essential in reducing the likelihood of tumor formation []. The potential of statins to mitigate the risk of meningioma has been the subject of divergent findings in prior research. For instance, an in vitro study by Gehring, et al. revealed that statins exert a cytotoxic effect on meningioma cells, hinting at their possible application in treating meningiomas []. Conversely, in a case-control study conducted by Seliger, et al. no connection was found between the use of statins and the risk of meningioma []. Acknowledging the challenges previous studies faced in eliminating confounding factors and drawing reliable conclusions, our Mendelian randomization analysis introduces a novel perspective and methodology. Using genetic variations as instrumental variables allows
for a more accurate assessment of the causal link between drug targets and disease, confirming the effectiveness of statins in reducing the likelihood of malignant meningiomas.
Our study is not without limitations. The limitations of utilizing genetic variations to examine the impacts of lipid-lowering medications are evident, given the minimal genetic influence that emerges gradually, contrasting with the more pronounced effects of drug treatments within distinct time frames. Therefore, analyzing drug-target interactions may not accurately reflect the immediate impact of lipid-lowering medications. Secondly, our MR analysis might be limited by low statistical power, as evidenced by the confidence intervals of the MR estimates. Thirdly, genetic variants used to demonstrate exposure could introduce bias into the MR results through pleiotropy. Furthermore, as this research relied on information gathered from a European demographic, the findings may not be generalizable to other racial groups. Fifthly, the analysis did not consider interactions between genetic variants and targeted drugs. Lastly, this analysis only revealed the precise effects of drug use, neglecting the non-target consequences of related medications.
The study used eQTLs for drug target genes (HMGCR, PCSK9, NPC1L1, and APOB) as substitutes to represent exposure to lipid-lowering drugs. eQTL analysis, by examining the relationship between genomic variations and gene expression intensity across a large sample size, emerges as a crucial approach to uncovering the functional impact of genomic variations on genes from a genome-wide perspective. It serves as an essential tool for revealing the relationships between genetic variants, genes, and phenotypes, where improving eQTL detection efficiency is beneficial for discovering new regulatory elements and target genes, playing a significant role in understanding genetic regulatory mechanisms []. The SMR method is used with eQTLs as instruments to estimate effects, examining the connection between gene expression levels and outcomes by analyzing summary data from GWAS and eQTL studies [].
To sum up, this study used genetic markers linked to HMGCR levels or HMGCR-controlled LDL cholesterol as proxies for statin use and found that HMGCR blockers could potentially lower the chances of developing malignant meningiomas.MR studies, as a genetic epidemiological approach, have the ability to address the constraints of conventional observational studies. In upcoming clinical trials focused on lowering the risk of meningiomas, there may be a preference for statins, representing a notable advancement in repurposing current drugs for novel therapeutic uses.
In our research, we employed Mendelian randomization to examine how lipid-lowering medications, such as statins, impact the likelihood of developing malignant meningiomas. The results indicate that statins could provide a safeguarding benefit against this type of cancer. By leveraging genetic variants as instrumental variables for statin exposure, we provide new insights that could lead to repurposing existing medications for the prevention of malignant meningiomas. Despite certain limitations, such as the potential for ethnic variability in genetic impacts, these results underscore the value of genetic epidemiology in identifying new therapeutic strategies and highlight the potential of statins as a preventive measure against malignant meningiomas in future clinical trials.
Funding: This study was funded by the national college student scientific research project (Project No.2023004).
Authors’ contributions: The study’s planning and manuscript writing were done by Liantai Song and Xiaoyan Guo. The data was collected and examined by Wenhui Zhang, Mengjie Li, and Xinyi Wu. The final paper was examined and approved by Ziqian Kou, Yuxin Wang, and Zigeng Ren. Qian Xu oversaw all aspects of the project, including data interpretation and article review. The final draft of the paper was approved by all authors after they had evaluated the findings.
Data availability statement: The eQTL data used in this study were downloaded from the eQTLGen (https://eqtlgen.org/) and the Genotype-Tissue Expression (GTEx) project V.8 (https://gtexportal.org/home/datasets). The genome-wide association study (GWAS) data for LDL cholesterol, Malignant meningioma, and Coronary atherosclerosis were obtained from the IEU Open GWAS database (https://gwas.mrcieu.ac.uk/).
Ethics approval and consent to participate: This Mendelian Randomization (MR) study utilizes publicly available summary-level data from Genome-Wide Association Studies (GWASs) and expression quantitative trait loci (eQTLs) studies. All the original studies from which this data was sourced received approval from the relevant institutional review boards, and all participants had provided informed consent. The data used in this study have been ethically approved and informed consent has been obtained from the participants in the original research.
Ostrom QT, Price M, Neff C, Cioffi G, Waite KA, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015-2019. Neuro Oncol. 2022 Oct 5;24(Suppl 5):v1-v95. doi: 10.1093/neuonc/noac202. PMID: 36196752; PMCID: PMC9533228.
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016 Jun;131(6):803-20. doi: 10.1007/s00401-016-1545-1. Epub 2016 May 9. PMID: 27157931.
Harter PN, Braun Y, Plate KH. Classification of meningiomas-advances and controversies. Chin Clin Oncol. 2017 Jul;6(Suppl 1):S2. doi: 10.21037/cco.2017.05.02. Epub 2017 Jun 4. PMID: 28595423.
Li Y, Drappatz J. Advances in the systemic therapy for recurrent meningiomas and the challenges ahead. Expert Rev Neurother. 2023 Jul-Dec;23(11):995-1004. doi: 10.1080/14737175.2023.2254498. Epub 2023 Sep 11. PMID: 37695700.
Johnson DR, Kimmel DW, Burch PA, Cascino TL, Giannini C, Wu W, Buckner JC. Phase II study of subcutaneous octreotide in adults with recurrent or progressive meningioma and meningeal hemangiopericytoma. Neuro Oncol. 2011 May;13(5):530-5. doi: 10.1093/neuonc/nor044. PMID: 21558077; PMCID: PMC3093340.
Ji Y, Rankin C, Grunberg S, Sherrod AE, Ahmadi J, Townsend JJ, Feun LG, Fredericks RK, Russell CA, Kabbinavar FF, Stelzer KJ, Schott A, Verschraegen C. Double-Blind Phase III Randomized Trial of the Antiprogestin Agent Mifepristone in the Treatment of Unresectable Meningioma: SWOG S9005. J Clin Oncol. 2015 Dec 1;33(34):4093-8. doi: 10.1200/JCO.2015.61.6490. Epub 2015 Nov 2. PMID: 26527781; PMCID: PMC4669593.
Anderson TR, Slotkin TA. Maturation of the adrenal medulla--IV. Effects of morphine. Biochem Pharmacol. 1975 Aug 15;24(16):1469-74. doi: 10.1016/0006-2952(75)90020-9. PMID: 7.
Vona R, Iessi E, Matarrese P. Role of Cholesterol and Lipid Rafts in Cancer Signaling: A Promising Therapeutic Opportunity? Front Cell Dev Biol. 2021 Mar 19; 9:622908. doi: 10.3389/fcell.2021.622908. PMID: 33816471; PMCID: PMC8017202.
Ding X, Zhang W, Li S, Yang H. The role of cholesterol metabolism in cancer. Am J Cancer Res. 2019 Feb 1;9(2):219-227. PMID: 30906624; PMCID: PMC6405981.
Mayengbam SS, Singh A, Pillai AD, Bhat MK. Influence of cholesterol on cancer progression and therapy. Transl Oncol. 2021 Jun;14(6):101043. doi: 10.1016/j.tranon.2021.101043. Epub 2021 Mar 20. PMID: 33751965; PMCID: PMC8010885.
Vogel FCE, Chaves-Filho AB, Schulze A. Lipids as mediators of cancer progression and metastasis. Nat Cancer. 2024 Jan;5(1):16-29. doi: 10.1038/s43018-023-00702-z. Epub 2024 Jan 25. PMID: 38273023.
Son M, Baek A, Sakkiah S, Park C, John S, Lee KW. Exploration of virtual candidates for human HMG-CoA reductase inhibitors using pharmacophore modeling and molecular dynamics simulations. PLoS One. 2013 Dec 30; 8(12):e83496. doi: 10.1371/journal.pone.0083496. PMID: 24386216; PMCID: PMC3875450.
Xia XD, Peng ZS, Gu HM, Wang M, Wang GQ, Zhang DW. Regulation of PCSK9 Expression and Function: Mechanisms and Therapeutic Implications. Front Cardiovasc Med. 2021 Oct 15; 8:764038. doi: 10.3389/fcvm.2021.764038. PMID: 34782856; PMCID: PMC8589637.
Zhang R, Zeng J, Liu W, Meng J, Wang C, Shi L, Yang S, Chang J, Xing D. The role of NPC1L1 in cancer. Front Pharmacol. 2022 Aug 10; 13:956619. doi: 10.3389/fphar.2022.956619. PMID: 36034854; PMCID: PMC9399402.
Behbodikhah J, Ahmed S, Elyasi A, Kasselman LJ, De Leon J, Glass AD, Reiss AB. Apolipoprotein B and Cardiovascular Disease: Biomarker and Potential Therapeutic Target. Metabolites. 2021 Oct 8;11(10):690. doi: 10.3390/metabo11100690. PMID: 34677405; PMCID: PMC8540246.
Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, Laurin C, Burgess S, Bowden J, Langdon R, Tan VY, Yarmolinsky J, Shihab HA, Timpson NJ, Evans DM, Relton C, Martin RM, Davey Smith G, Gaunt TR, Haycock PC. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018 May 30; 7:e34408. doi: 10.7554/eLife.34408. PMID: 29846171; PMCID: PMC5976434.
Schmidt AF, Finan C, Gordillo-Marañón M, Asselbergs FW, Freitag DF, Patel RS, Tyl B, Chopade S, Faraway R, Zwierzyna M, Hingorani AD. Genetic drug target validation using Mendelian randomisation. Nat Commun. 2020 Jun 26;11(1):3255. doi: 10.1038/s41467-020-16969-0. PMID: 32591531; PMCID: PMC7320010.
Wang K, Shi M, Huang C, Fan B, Luk AOY, Kong APS, Ma RCW, Chan JCN, Chow E. Evaluating the impact of glucokinase activation on risk of cardiovascular disease: a Mendelian randomisation analysis. Cardiovasc Diabetol. 2022 Sep 23;21(1):192. doi: 10.1186/s12933-022-01613-6. PMID: 36151532; PMCID: PMC9503210.
Ueda M, Fukui K, Kamatani N, Kamitsuji S, Matsuo A, Sasase T, Nishiu J, Matsushita M. GLUT9 as a potential drug target for chronic kidney disease: Drug target validation by a Mendelian randomization study. J Hum Genet. 2023 Oct;68(10):699-704. doi: 10.1038/s10038-023-01168-8. Epub 2023 Jun 13. PMID: 37308567.
Willer CJ, Schmidt EM, Sengupta S, Peloso GM, Gustafsson S, et al. Global Lipids Genetics Consortium. Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013 Nov;45(11):1274-1283. doi: 10.1038/ng.2797. Epub 2013 Oct 6. PMID: 24097068; PMCID: PMC3838666.
Kurki MI. et al. editors. FinnGen: Unique genetic insights from combining isolated population and national health register data. medRxiv; 2022.
Zhu Z, Zhang F, Hu H, Bakshi A, Robinson MR, Powell JE, Montgomery GW, Goddard ME, Wray NR, Visscher PM, Yang J. Integration of summary data from GWAS and eQTL studies predicts complex trait gene targets. Nat Genet. 2016 May;48(5):481-7. doi: 10.1038/ng.3538. Epub 2016 Mar 28. PMID: 27019110.
Burgess S, Thompson SG; CRP CHD Genetics Collaboration. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. 2011 Jun;40(3):755-64. doi: 10.1093/ije/dyr036. Epub 2011 Mar 16. PMID: 21414999.
Chauquet S, Zhu Z, O'Donovan MC, Walters JTR, Wray NR, Shah S. Association of Antihypertensive Drug Target Genes with Psychiatric Disorders: A Mendelian Randomization Study. JAMA Psychiatry. 2021 Jun 1;78(6):623-631. doi: 10.1001/jamapsychiatry.2021.0005. PMID: 33688928; PMCID: PMC7948097.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003 Sep 6;327(7414):557-60. doi: 10.1136/bmj.327.7414.557. PMID: 12958120; PMCID: PMC192859.
Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017 May;32(5):377-389. doi: 10.1007/s10654-017-0255-x. Epub 2017 May 19. Erratum in: Eur J Epidemiol. 2017 Jun 29; PMID: 28527048; PMCID: PMC5506233.
Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018 May;50(5):693-698. doi: 10.1038/s41588-018-0099-7. Epub 2018 Apr 23. Erratum in: Nat Genet. 2018 Aug;50(8):1196. PMID: 29686387; PMCID: PMC6083837.
Apra C, Peyre M, Kalamarides M. Current treatment options for meningioma. Expert Rev Neurother. 2018 Mar;18(3):241-249. doi: 10.1080/14737175.2018.1429920. Epub 2018 Jan 22. PMID: 29338455.
Sughrue ME, Sanai N, Shangari G, Parsa AT, Berger MS, McDermott MW. Outcome and survival following primary and repeat surgery for World Health Organization Grade III meningiomas. J Neurosurg. 2010 Aug;113(2):202-9. doi: 10.3171/2010.1.JNS091114. PMID: 20225922.
Bergner A, Maier AD, Mirian C, Mathiesen TI. Adjuvant radiotherapy and stereotactic radiosurgery in grade 3 meningiomas - a systematic review and meta-analysis. Neurosurg Rev. 2022 Aug;45(4):2639-2658. doi: 10.1007/s10143-022-01773-9. Epub 2022 May 11. PMID: 35543810.
Drappatz J. How useful is chemotherapy for atypical and anaplastic meningiomas? Expert Opin Pharmacother. 2022 Oct;23(14):1559-1561. doi: 10.1080/14656566.2022.2131394. Epub 2022 Oct 5. PMID: 36189940.
Ahmeti H, Caliebe A, Röcken C, Jansen O, Mehdorn MH, Synowitz M. Impact of peritumoral brain edema on pre- and postoperative clinical conditions and on long-term outcomes in patients with intracranial meningiomas. Eur J Med Res. 2023 Jan 21;28(1):40. doi: 10.1186/s40001-022-00962-y. PMID: 36670509; PMCID: PMC9862965.
Combs SE, Edler L, Burkholder I, Rieken S, Habermehl D, Jäkel O, Haberer T, Unterberg A, Wick W, Debus J, Haselmann R. Treatment of patients with atypical meningiomas Simpson grade 4 and 5 with a carbon ion boost in combination with postoperative photon radiotherapy: the MARCIE trial. BMC Cancer. 2010 Nov 9; 10:615. doi: 10.1186/1471-2407-10-615. PMID: 21062428; PMCID: PMC2996393.
Zeng Q, Shi F, Guo Z. Effectiveness of Postoperative Radiotherapy on Atypical Meningioma Patients: A Population-Based Study. Front Oncol. 2019 Jan 31; 9:34. doi: 10.3389/fonc.2019.00034. PMID: 30805304; PMCID: PMC6371043.
Ward NC, Watts GF, Eckel RH. Statin Toxicity. Circ Res. 2019 Jan 18;124(2):328-350. doi: 10.1161/CIRCRESAHA.118.312782. PMID: 30653440.
Costet P. Molecular pathways and agents for lowering LDL-cholesterol in addition to statins. Pharmacol Ther. 2010 Jun;126(3):263-78. doi: 10.1016/j.pharmthera.2010.02.006. Epub 2010 Mar 19. PMID: 20227438.
Volpe M, Patrono C. The cardiovascular benefits of statins outweigh adverse effects in primary prevention: results of a large systematic review and meta-analysis. Eur Heart J. 2021 Nov 21;42(44):4518-4519. doi: 10.1093/eurheartj/ehab647. PMID: 34849717.
Cai T, Abel L, Langford O, Monaghan G, Aronson JK, Stevens RJ, Lay-Flurrie S, Koshiaris C, McManus RJ, Hobbs FDR, Sheppard JP. Associations between statins and adverse events in primary prevention of cardiovascular disease: systematic review with pairwise, network, and dose-response meta-analyses. BMJ. 2021 Jul 14; 374:n1537. doi: 10.1136/bmj.n1537. PMID: 34261627; PMCID: PMC8279037.
McFarlane SI, Muniyappa R, Francisco R, Sowers JR. Clinical review 145: Pleiotropic effects of statins: lipid reduction and beyond. J Clin Endocrinol Metab. 2002 Apr;87(4):1451-8. doi: 10.1210/jcem.87.4.8412. PMID: 11932263.
Gehring S, Tapia-Pérez JH, Kirches E, Firsching R, Keilhoff G, Schneider T, Mawrin C. Cytotoxic effects of statins and thiazolidinediones on meningioma cells. J Neurooncol. 2011 May;102(3):383-93. doi: 10.1007/s11060-010-0351-1. Epub 2010 Aug 30. PMID: 20803306.
Seliger C, Meier CR, Becker C, Jick SS, Proescholdt M, Bogdahn U, Hau P, Leitzmann MF. Metabolic syndrome in relation to risk of meningioma. Oncotarget. 2017 Jan 10;8(2):2284-2292. doi: 10.18632/oncotarget.13667. PMID: 27903988; PMCID: PMC5356799.
Song L, Guo X, Zhang W, Li M, Wu X, Kou Z, Wang Y, Ren Z, Xu Q. Examining the Causal Connection between Lipid-lowering Medications and Malignant Meningiomas through Drug-target Mendelian Randomization Analysis. IgMin Res. May 15, 2024; 2(5): 357-363. IgMin ID: igmin187; DOI:10.61927/igmin187; Available at: igmin.link/p187
次のリンクを共有した人は、このコンテンツを読むことができます:
Address Correspondence:
Qian Xu, Chengde Medical University, Chengde 067000, China, Email: [email protected]
How to cite this article:
Song L, Guo X, Zhang W, Li M, Wu X, Kou Z, Wang Y, Ren Z, Xu Q. Examining the Causal Connection between Lipid-lowering Medications and Malignant Meningiomas through Drug-target Mendelian Randomization Analysis. IgMin Res. May 15, 2024; 2(5): 357-363. IgMin ID: igmin187; DOI:10.61927/igmin187; Available at: igmin.link/p187
Copyright: © 2024 Song L, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
 Figure 1: Using summary data, a Mendelian Randomization anal...
Figure 1: Using summary data, a Mendelian Randomization anal...
 Figure 2: Results from the Inverse-Variance Weighted (IVW) m...
Figure 2: Results from the Inverse-Variance Weighted (IVW) m...
Ostrom QT, Price M, Neff C, Cioffi G, Waite KA, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015-2019. Neuro Oncol. 2022 Oct 5;24(Suppl 5):v1-v95. doi: 10.1093/neuonc/noac202. PMID: 36196752; PMCID: PMC9533228.
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016 Jun;131(6):803-20. doi: 10.1007/s00401-016-1545-1. Epub 2016 May 9. PMID: 27157931.
Harter PN, Braun Y, Plate KH. Classification of meningiomas-advances and controversies. Chin Clin Oncol. 2017 Jul;6(Suppl 1):S2. doi: 10.21037/cco.2017.05.02. Epub 2017 Jun 4. PMID: 28595423.
Li Y, Drappatz J. Advances in the systemic therapy for recurrent meningiomas and the challenges ahead. Expert Rev Neurother. 2023 Jul-Dec;23(11):995-1004. doi: 10.1080/14737175.2023.2254498. Epub 2023 Sep 11. PMID: 37695700.
Johnson DR, Kimmel DW, Burch PA, Cascino TL, Giannini C, Wu W, Buckner JC. Phase II study of subcutaneous octreotide in adults with recurrent or progressive meningioma and meningeal hemangiopericytoma. Neuro Oncol. 2011 May;13(5):530-5. doi: 10.1093/neuonc/nor044. PMID: 21558077; PMCID: PMC3093340.
Ji Y, Rankin C, Grunberg S, Sherrod AE, Ahmadi J, Townsend JJ, Feun LG, Fredericks RK, Russell CA, Kabbinavar FF, Stelzer KJ, Schott A, Verschraegen C. Double-Blind Phase III Randomized Trial of the Antiprogestin Agent Mifepristone in the Treatment of Unresectable Meningioma: SWOG S9005. J Clin Oncol. 2015 Dec 1;33(34):4093-8. doi: 10.1200/JCO.2015.61.6490. Epub 2015 Nov 2. PMID: 26527781; PMCID: PMC4669593.
Anderson TR, Slotkin TA. Maturation of the adrenal medulla--IV. Effects of morphine. Biochem Pharmacol. 1975 Aug 15;24(16):1469-74. doi: 10.1016/0006-2952(75)90020-9. PMID: 7.
Vona R, Iessi E, Matarrese P. Role of Cholesterol and Lipid Rafts in Cancer Signaling: A Promising Therapeutic Opportunity? Front Cell Dev Biol. 2021 Mar 19; 9:622908. doi: 10.3389/fcell.2021.622908. PMID: 33816471; PMCID: PMC8017202.
Ding X, Zhang W, Li S, Yang H. The role of cholesterol metabolism in cancer. Am J Cancer Res. 2019 Feb 1;9(2):219-227. PMID: 30906624; PMCID: PMC6405981.
Mayengbam SS, Singh A, Pillai AD, Bhat MK. Influence of cholesterol on cancer progression and therapy. Transl Oncol. 2021 Jun;14(6):101043. doi: 10.1016/j.tranon.2021.101043. Epub 2021 Mar 20. PMID: 33751965; PMCID: PMC8010885.
Vogel FCE, Chaves-Filho AB, Schulze A. Lipids as mediators of cancer progression and metastasis. Nat Cancer. 2024 Jan;5(1):16-29. doi: 10.1038/s43018-023-00702-z. Epub 2024 Jan 25. PMID: 38273023.
Son M, Baek A, Sakkiah S, Park C, John S, Lee KW. Exploration of virtual candidates for human HMG-CoA reductase inhibitors using pharmacophore modeling and molecular dynamics simulations. PLoS One. 2013 Dec 30; 8(12):e83496. doi: 10.1371/journal.pone.0083496. PMID: 24386216; PMCID: PMC3875450.
Xia XD, Peng ZS, Gu HM, Wang M, Wang GQ, Zhang DW. Regulation of PCSK9 Expression and Function: Mechanisms and Therapeutic Implications. Front Cardiovasc Med. 2021 Oct 15; 8:764038. doi: 10.3389/fcvm.2021.764038. PMID: 34782856; PMCID: PMC8589637.
Zhang R, Zeng J, Liu W, Meng J, Wang C, Shi L, Yang S, Chang J, Xing D. The role of NPC1L1 in cancer. Front Pharmacol. 2022 Aug 10; 13:956619. doi: 10.3389/fphar.2022.956619. PMID: 36034854; PMCID: PMC9399402.
Behbodikhah J, Ahmed S, Elyasi A, Kasselman LJ, De Leon J, Glass AD, Reiss AB. Apolipoprotein B and Cardiovascular Disease: Biomarker and Potential Therapeutic Target. Metabolites. 2021 Oct 8;11(10):690. doi: 10.3390/metabo11100690. PMID: 34677405; PMCID: PMC8540246.
Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, Laurin C, Burgess S, Bowden J, Langdon R, Tan VY, Yarmolinsky J, Shihab HA, Timpson NJ, Evans DM, Relton C, Martin RM, Davey Smith G, Gaunt TR, Haycock PC. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018 May 30; 7:e34408. doi: 10.7554/eLife.34408. PMID: 29846171; PMCID: PMC5976434.
Schmidt AF, Finan C, Gordillo-Marañón M, Asselbergs FW, Freitag DF, Patel RS, Tyl B, Chopade S, Faraway R, Zwierzyna M, Hingorani AD. Genetic drug target validation using Mendelian randomisation. Nat Commun. 2020 Jun 26;11(1):3255. doi: 10.1038/s41467-020-16969-0. PMID: 32591531; PMCID: PMC7320010.
Wang K, Shi M, Huang C, Fan B, Luk AOY, Kong APS, Ma RCW, Chan JCN, Chow E. Evaluating the impact of glucokinase activation on risk of cardiovascular disease: a Mendelian randomisation analysis. Cardiovasc Diabetol. 2022 Sep 23;21(1):192. doi: 10.1186/s12933-022-01613-6. PMID: 36151532; PMCID: PMC9503210.
Ueda M, Fukui K, Kamatani N, Kamitsuji S, Matsuo A, Sasase T, Nishiu J, Matsushita M. GLUT9 as a potential drug target for chronic kidney disease: Drug target validation by a Mendelian randomization study. J Hum Genet. 2023 Oct;68(10):699-704. doi: 10.1038/s10038-023-01168-8. Epub 2023 Jun 13. PMID: 37308567.
Willer CJ, Schmidt EM, Sengupta S, Peloso GM, Gustafsson S, et al. Global Lipids Genetics Consortium. Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013 Nov;45(11):1274-1283. doi: 10.1038/ng.2797. Epub 2013 Oct 6. PMID: 24097068; PMCID: PMC3838666.
Kurki MI. et al. editors. FinnGen: Unique genetic insights from combining isolated population and national health register data. medRxiv; 2022.
Zhu Z, Zhang F, Hu H, Bakshi A, Robinson MR, Powell JE, Montgomery GW, Goddard ME, Wray NR, Visscher PM, Yang J. Integration of summary data from GWAS and eQTL studies predicts complex trait gene targets. Nat Genet. 2016 May;48(5):481-7. doi: 10.1038/ng.3538. Epub 2016 Mar 28. PMID: 27019110.
Burgess S, Thompson SG; CRP CHD Genetics Collaboration. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. 2011 Jun;40(3):755-64. doi: 10.1093/ije/dyr036. Epub 2011 Mar 16. PMID: 21414999.
Chauquet S, Zhu Z, O'Donovan MC, Walters JTR, Wray NR, Shah S. Association of Antihypertensive Drug Target Genes with Psychiatric Disorders: A Mendelian Randomization Study. JAMA Psychiatry. 2021 Jun 1;78(6):623-631. doi: 10.1001/jamapsychiatry.2021.0005. PMID: 33688928; PMCID: PMC7948097.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003 Sep 6;327(7414):557-60. doi: 10.1136/bmj.327.7414.557. PMID: 12958120; PMCID: PMC192859.
Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017 May;32(5):377-389. doi: 10.1007/s10654-017-0255-x. Epub 2017 May 19. Erratum in: Eur J Epidemiol. 2017 Jun 29; PMID: 28527048; PMCID: PMC5506233.
Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018 May;50(5):693-698. doi: 10.1038/s41588-018-0099-7. Epub 2018 Apr 23. Erratum in: Nat Genet. 2018 Aug;50(8):1196. PMID: 29686387; PMCID: PMC6083837.
Apra C, Peyre M, Kalamarides M. Current treatment options for meningioma. Expert Rev Neurother. 2018 Mar;18(3):241-249. doi: 10.1080/14737175.2018.1429920. Epub 2018 Jan 22. PMID: 29338455.
Sughrue ME, Sanai N, Shangari G, Parsa AT, Berger MS, McDermott MW. Outcome and survival following primary and repeat surgery for World Health Organization Grade III meningiomas. J Neurosurg. 2010 Aug;113(2):202-9. doi: 10.3171/2010.1.JNS091114. PMID: 20225922.
Bergner A, Maier AD, Mirian C, Mathiesen TI. Adjuvant radiotherapy and stereotactic radiosurgery in grade 3 meningiomas - a systematic review and meta-analysis. Neurosurg Rev. 2022 Aug;45(4):2639-2658. doi: 10.1007/s10143-022-01773-9. Epub 2022 May 11. PMID: 35543810.
Drappatz J. How useful is chemotherapy for atypical and anaplastic meningiomas? Expert Opin Pharmacother. 2022 Oct;23(14):1559-1561. doi: 10.1080/14656566.2022.2131394. Epub 2022 Oct 5. PMID: 36189940.
Ahmeti H, Caliebe A, Röcken C, Jansen O, Mehdorn MH, Synowitz M. Impact of peritumoral brain edema on pre- and postoperative clinical conditions and on long-term outcomes in patients with intracranial meningiomas. Eur J Med Res. 2023 Jan 21;28(1):40. doi: 10.1186/s40001-022-00962-y. PMID: 36670509; PMCID: PMC9862965.
Combs SE, Edler L, Burkholder I, Rieken S, Habermehl D, Jäkel O, Haberer T, Unterberg A, Wick W, Debus J, Haselmann R. Treatment of patients with atypical meningiomas Simpson grade 4 and 5 with a carbon ion boost in combination with postoperative photon radiotherapy: the MARCIE trial. BMC Cancer. 2010 Nov 9; 10:615. doi: 10.1186/1471-2407-10-615. PMID: 21062428; PMCID: PMC2996393.
Zeng Q, Shi F, Guo Z. Effectiveness of Postoperative Radiotherapy on Atypical Meningioma Patients: A Population-Based Study. Front Oncol. 2019 Jan 31; 9:34. doi: 10.3389/fonc.2019.00034. PMID: 30805304; PMCID: PMC6371043.
Ward NC, Watts GF, Eckel RH. Statin Toxicity. Circ Res. 2019 Jan 18;124(2):328-350. doi: 10.1161/CIRCRESAHA.118.312782. PMID: 30653440.
Costet P. Molecular pathways and agents for lowering LDL-cholesterol in addition to statins. Pharmacol Ther. 2010 Jun;126(3):263-78. doi: 10.1016/j.pharmthera.2010.02.006. Epub 2010 Mar 19. PMID: 20227438.
Volpe M, Patrono C. The cardiovascular benefits of statins outweigh adverse effects in primary prevention: results of a large systematic review and meta-analysis. Eur Heart J. 2021 Nov 21;42(44):4518-4519. doi: 10.1093/eurheartj/ehab647. PMID: 34849717.
Cai T, Abel L, Langford O, Monaghan G, Aronson JK, Stevens RJ, Lay-Flurrie S, Koshiaris C, McManus RJ, Hobbs FDR, Sheppard JP. Associations between statins and adverse events in primary prevention of cardiovascular disease: systematic review with pairwise, network, and dose-response meta-analyses. BMJ. 2021 Jul 14; 374:n1537. doi: 10.1136/bmj.n1537. PMID: 34261627; PMCID: PMC8279037.
McFarlane SI, Muniyappa R, Francisco R, Sowers JR. Clinical review 145: Pleiotropic effects of statins: lipid reduction and beyond. J Clin Endocrinol Metab. 2002 Apr;87(4):1451-8. doi: 10.1210/jcem.87.4.8412. PMID: 11932263.
Gehring S, Tapia-Pérez JH, Kirches E, Firsching R, Keilhoff G, Schneider T, Mawrin C. Cytotoxic effects of statins and thiazolidinediones on meningioma cells. J Neurooncol. 2011 May;102(3):383-93. doi: 10.1007/s11060-010-0351-1. Epub 2010 Aug 30. PMID: 20803306.
Seliger C, Meier CR, Becker C, Jick SS, Proescholdt M, Bogdahn U, Hau P, Leitzmann MF. Metabolic syndrome in relation to risk of meningioma. Oncotarget. 2017 Jan 10;8(2):2284-2292. doi: 10.18632/oncotarget.13667. PMID: 27903988; PMCID: PMC5356799.