
The Expressivity Dimension of Speech is the basis of the Expression Dimension. Evidence from Behavioural and Neuroimaging Studies
Biomedicine BiophysicsBiology受け取った 13 Mar 2024 受け入れられた 03 May 2024 オンラインで公開された 06 May 2024
Focusing on Biology, Medicine and Engineering ISSN: 2995-8067 | Quick Google Scholar
Next Full Text
New Scientific Field for Modelling Complex Dynamical Systems: The Cybernetics Artificial Intelligence (CAI)
Previous Full Text
Peritoneal Carcinomatosis from Ovarian Cancer: A Case Report


受け取った 13 Mar 2024 受け入れられた 03 May 2024 オンラインで公開された 06 May 2024
The modalities of communication are the sum of the expression dimension (linguistics) and the expressivity dimension (prosody), both being equally important in language communication. The expressivity dimension which comes first in the act of speech, is the basis on which phonemes, syllables, words, grammar, and morphosyntax, i.e., the expression dimension of speech is superimposed. We will review evidence (1) revealing the importance of prosody in language acquisition and (2) showing that prosody triggers the involvement of specific brain areas dedicated to sentences and word-list processing. To support the first point, we will not only rely on experimental psychology studies conducted in newborns and young children but also on neuroimaging studies that have helped to validate these behavioral experiments. Then, neuroimaging data on adults will allow for the conclusion that the expressivity dimension of speech modulates both the right hemisphere prosodic areas and the left hemisphere network in charge of the expression dimension.
Linguistics covers many fields such as phonetics, phonology, morphology, semantics, and grammar... Numerous schools have thus emerged, each focusing on one or more aspects, which enables them to achieve great precision in their field of investigation. The focus of these studies is generally on language, that is the verbal modalities of communication, and not on “speech”; while the modalities of communication can be represented by the sum of verbal and non-verbal modalities, these two components being equally important in language communication (Figure 1). Dealing with speech and language, one often thinks of the “expression” dimension, leaving aside the “expressivity” dimension, i.e. the prosodic dimension, which is even considered at times as supra-segmental. However, speech is perceived and produced in a global way: intellectual meaning (content of expression) and affective meaning (content of expressivity) are perceived and produced at the same time.
The expressivity dimension is made up of both kinesic and prosodic aspects of speech, prosody, i.e., the music of speech, being the topic of our discussion. Prosody is mainly characterised by the variations of the fundamental frequency (F0), which is the number per second of the closures and openings of the vocal cords situated in the larynx. The intensity of the voice is essentially due to the amplitude of the vibrations of the vocal cords and the subglottic pressure exerted by the air coming from the lungs. The duration, or rhythm of the utterance, concerns the temporal organization of the message and includes the speech rate (number of syllables spoken in a given unit of time) and tempo (the pauses and acceleration or deceleration of the rate within a prosodic group).
The expressivity dimension, which can be compared to a musical stave, comes first in the act of speech; in other words, it is the basis on which phonemes, syllables, words, grammar, and morphosyntax, i.e., the expression dimension of speech is superimposed. Evidence (1) revealing the importance of prosody in language acquisition and (2) showing that prosody triggers the involvement of specific brain areas dedicated to sentences and word-list processing will be reviewed. To support the first point, experimental psychology studies conducted in newborns and young children but also on neuroimaging studies that have helped to validate these behavioural experiments will be addressed. The second point about adults will focus on the neuroimaging data.
Prosody and speech perception: From a phonetic point of view, prosody describes the variations in the pitch, intensity, and duration of utterances [] which correspond perceptually to the phenomena of intonation, accentuation, speech rate, or the perception of pauses. These phenomena have different functions: pragmatic, modal, syntactic, and expressive, allowing babies to pre-segment the sound rate into units of sound and meaning, and to acquire the lexicon of their language [-]. From a perceptive point of view, prosody is the first language structure perceived by children [,], and from a productive point of view, prosody is the first early functional system [,]. The prosodic organization of human communication has been reported to be continuous and highly correlated with the phonological [] semantic [] syntactic [] morphological, and segmental organization of speech []. Prior to birth, the hearing system is already functional during the last trimester of gestation. In utero, the rhythmic and prosodic information of the mother’s speech is transmitted to the inner ear of the foetus by bone conduction, allowing her/ him or her to learn the properties of the mother tongue. This prenatal speech input is filtered by maternal tissues and propagates through fluid. Animal models and computational simulations reveal that this input is low-passed filtered at around 300–400 Hz, which mainly preserves the fundamental frequency, and thus the rhythm and the intensity of the signal and voicing information contrary to the place and manner of articulation information which is not preserved [-]. Interestingly, a behavioural study using the nonnutritive sucking technique has revealed that newborns prefer a low-pass filtered version of the maternal voice to an unfiltered version []. Using the same technique, other studies have shown that newborn babies give signs of a preference for their mother’s voice a few hours after birth [,]. Some studies have also revealed that newborn babies show a physiological response to the maternal voice since they exhibit heart rate decelerations [] and fewer movements [] while listening to a recording of their mother’s voice than when listening to a stranger female voice []. Taken together, it appears that speech heard in utero starts shaping infants’ perceptual abilities and brain specialization for speech before birth []. However, this remains really complex to investigate behaviourally.
That is why some neuroimaging studies supporting behavioural findings have been conducted on healthy preterm infants. After the 30th week of gestation, the foetal auditory system is mature enough to detect speech sounds as reported by an EventRelated Potentials (ERPs) study [] and to differentiate phonemes []. A functional Magnetic Resonance Imagery (fMRI) study has revealed that foetuses between 33 and 34 weeks of gestation present more activation in the left temporal lobe when they listen to the maternal voice as compared to other female voices or to pure tones []. In addition, the foetal auditory system is able to differentiate a familiar language from an unfamiliar one, showing that even before birth, the foetus is listening to his/her linguistic environment as evidenced by a Near InfraRed Spectroscopy (NIRS) study []. Some studies have investigated newborn babies’ perceptual abilities, inferring that their performances at birth reveal that the mechanisms at stake were already active in utero. Hence, an ERP study at birth has revealed that neural memory traces are formed by auditory learning prior to birth []. Using optical topography in neonates, a study has revealed that the Left Hemisphere (LH) temporal areas show significantly more activation when infants are exposed to normal speech than to backward speech or silence []. The theory of early sensitivity to prosodic cues is also supported by the observation of decreased neural activity in response to speech stimuli presenting distorted prosody [,]. These brain imaging functional studies are sustained by structural Magnetic Resonance Imagery (MRI) studies revealing leftward asymmetries in language areas at birth []. Saito, et al. [] used near-infrared spectroscopy (NIRS) to demonstrate that newborns are able to discriminate between different prosodic patterns []. The newborn’s perceptive abilities, based on prosodic cues, enable him/her to recognise the mother’s voice and then to discriminate the inflections of the human voice that correspond to an interpretable semantic content. He is not only able to distinguish his mother tongue from a foreign language but also to differentiate between two foreign languages (provided they belong to different rhythmic classes), [,,]. At 4 months of age, the baby is able to discriminate syllables differing by a single acoustic index (voicing in the case of /pa/ vs. /ba/) and to distinguish male from female vocal productions [-]. In addition, young children use prosodic information to distinguish the grammatical category of words [] and to relate phonology and syntax [,]. Seeking to investigate the neural mechanisms underpinning speech perception in young babies, an fMRI study has revealed that the left-lateralized superior temporal and angular gyri are active in 3 months old babies’ brains listening to their mother tongue just as they are in adults []. Moreover, this study evidenced a rightward activation in the prefrontal cortex indicating the early engagement of active memory retrieval mechanisms, just as this region is recruited when adults retrieve verbal information from memory [,]. This suggests that 3-month-old infants have already memorized the prosodic contours of their native language, these prosodic contours being the basis on which the lexis is superimposed since they may not remember single words until the age of 7 months.
Prosody and speech production: Babies under one year of age already communicate with their bodies, their looks, gestures, intonations, babbling, chattering, and rhythms, which represent the stages preceding speech and which could be described as pre-speech manifestations. Altogether, the situation, the rhythm, and the intonation make the understanding between the adult and the baby under one year old possible. Some studies have looked at the evolution of the prosodic dimension in children’s oral productions. Newborns’ cries match the melody of their native language: French newborns’ cries present a melody that rises slowly and then quickly decreases, whereas the melody of German newborns’ cries rises quickly and slowly decreases []. At the age of 3 months, the children’s vocal programmes take on a differentiated character, even if they remain very rudimentary, few in number, and accompanied by shouting and growling [,]. Then, around the fourth month, the baby becomes more skilled at modulating and controlling the changes in pitch, duration, and intensity of his voice; he enters the “exploratory phase”, also called the “expansion phase” []. Vocalisation increases in quantity, variety, and complexity. This is the onset of the “vocal games” through which basic communication with the environment begins to take place []. At this point, prosody already carries communicative and reflexive functions, which are psychological processes indispensable to the emergence of language and its units.
From the age of 6-7 months, pre-linguistic productions are composed of prosodic properties specific to the mother tongue. The baby’s vocal activity is therefore rapidly influenced by the mother tongue, particularly by its rhythmic properties [,]. Then, from the second half of the first year of life, the child enters the actual babbling phase. This phase marks a break from the previous period. The child begins to make choices specific to the structures of his or her mother tongue, at the prosodic, phonetic, and syllabic levels [,,]. Babbling consists of a sequence of ordered steps. From 4 to 8 months of age, there are abrupt changes in the fundamental frequency, bitonal voice production, and voice tremor. The phonetic repertoire expands with the appearance of long-held consonant sounds. Around the age of 6 months, the “marginal babble” consists of consonant-vowel assemblages that are difficult to segment because of fairly loose articulation and very slow transitions between the closing and opening movements of the vocal tract.
Then, between 7 and 10 months of age, the “canonical babbling” appears, characterized by the production of single syllables (consonant-vowel: CV) very often reduced to “papapapa” or “mamamama” series. To express a request, a comment, etc., the child uses melodic patterns for linguistic purposes. The melody of the babbling then becomes intonation. The babbling will then become more diverse, with successive syllables differing from each other either by a consonant, vowel, or both (“patata”, “tokaba”, “badata”). In these series, the young child favours open syllables of the CV type over closed syllables: CVC [,] as well as a more diverse production of vowels ihman and Velleman, 2000; Vihman M., & Miller R., 1988) [,] that fill the intonational contours produced and constitute the proto-words, the future holophrases of children.
The strong implication of the prosodic dimension is already present in the first statements. Indeed, isolated words can exist as statements in their own right and be interpreted as such thanks to the prosody that conveys the illocutionary force of the message [-]. In addition, the same studies show that on the one hand, when children begin to produce their first words, they already display an extensive span of intonational contours and that the development of the intonational system is correlated with an increase in vocabulary span; while on the other hand, when children’s productions become identifiable as signifying units of the adult language, there occurs a kind of “reorganization” of the set of the intonational contours being produced; this can last until the end of the period of isolated words, or even a little longer. From that moment on, the descending contours, hitherto predominant, whatever the type of expression, begin to regress in favour of the ascending contours. Research has also highlighted the gradual introduction of the lengthening of the final syllable, which in some languages is intended to emphasize accentuation [,]. In French, for example, this phenomenon appears between the ages of 13 and 16 months, rapidly following the occurrence of the first words. The absence or exaggerated lengthening of the final syllable can therefore be considered as a potential marker of original or delayed development. Early word associations are also based on the prosodic dimension [,].
These first steps towards articulation are critical steps reflecting the functional link between the processes of perception and production of vocal sounds which gives the child the opportunity to process proprio-perceptual feedback []. Such a hypothesis has been validated by a MagnetoEncephaloGraphy (MEG) study revealing that the onset of imitation and canonical babbling around 5 months of age relies on the development of the connection between the auditory brain areas responsive to hearing speech and the brain areas involved in speech articulation (left Inferior Frontal Gyrus), providing evidence of a perceptual-motor link for speech perception that depends on experience []. In other words, infants can relate auditory and articulatory instantiations of speech [], and the brain mechanisms underlying speech perception and those controlling speech motor systems emerge over time as a function of experience [,]. Great deals of studies have reported that speech perception entails activation in speech production areas, more particularly in motor areas [-]. This can be related to the Motor Theory of Speech, which postulates that articulatory gestures support the development of speech production and perception, and activate a loop between the motor and perceptual areas [] whose brain support has been investigated with functional imaging.
In adults, a meta-analysis of brain imaging studies has revealed the existence of an elementary audio-motor loop involved in both comprehension and production of syllables, which includes the primary auditory areas and the motormouth area []. This loop is composed of Heschl’s gyrus and the planum temporale in the temporal lobe as its perceptive component and of the mouth motor area and inferior precentral cortex corresponding to the motor component []. This motor-sound–based representation of language sounds involves whether language is heard or enunciated, as proposed by Buschsbaum for auditory areas [] and by Wilson for motor areas [].
This audio-motor loop has been recently revealed to be at work very early, since 7 months old infants activate both the auditory (superior temporal areas) as well as the motor brain areas (frontal areas) during speech perception, whereas only the auditory areas are activated in newborns []; which supports the idea that exposure to native-language speech over the first 12 months of life produces neural changes not only in the auditory cortex but also in the brain regions that subserve articulation, and in those that allow a connection between the two systems.
All this taken together, we can propose that the perception and production of the Expressivity dimension come first in the course of language development and that the Expression dimension, first characterized by phonology, is intrinsically linked with the Expressivity dimension (Figure 2). However, one unresolved question concerns the involvement of the prosodic dimension in the phonological audio-motor loop.
At the sentence level: Understanding a language means being able to simultaneously master the linguistic, pragmatic, and affective structures of discourse, i.e. having a good command of the prosodic, phonological, semantic, and syntactic components that are intrinsically linked. So, language processing requires a widely distributed neural network involving unimodal, multimodal, and heteromodal areas, far beyond the traditional areas of Broca and Wernicke [,]. Even if most of the neuroimaging studies have explored the expression dimension, many studies seeking to determine the neural substrate underlying the perception and production of the prosodic dimension, i.e., the expressivity dimension, have demonstrated that the left perisylvian cortex is reported to play a major role in the treatment of linguistic prosody in most right-handed participants [-] and numerous studies have shown a strong right brain hemisphere involvement in the treatment of emotional prosody [-]. Some studies have shown that the right auditory cortex preferentially processes the fundamental frequency variations [-] which are one of the acoustic correlates of pitch variations and are considered very important cues for prosody processing [-], while bilateral recruitment has been demonstrated for the treatment of pitch per se [].
That is why we investigated the impact of the expressivity dimension on the expression dimension during listening, using 30 s connected speech stimuli of high degrees of prosodic information (pitch modulation ranging from 75 to 300 Hz) and low degrees of prosodic information (pitch modulation ranging from 75 to 150 Hz) in an fMRI study of 12 right-handed adults. High degrees of prosodic information do not only trigger right activations (such as the right inferior prefrontal cortex, the right supra temporal gyrus, and the right inferior parietal gyrus) but also trigger the involvement of the left dorsal pathway (the audio-motor loop) more than low degrees of prosodic information do []. As our two connected speech stimuli presented the same lexicosyntactic content, it can be concluded that the prosodic dimension influences the cooperation of both hemispheres. A really interesting result concerns the recruitment of this audio motor loop during the prosodic condition. More particularly, the activations of the SupraMarginal Gyrus (SMG) are consistent with the results of previous studies on speech perception. Indeed, the left SMG has been described as being involved in the processing of speech understanding and more specifically as being involved in both (1) the processing of auditory spatial information, [-]; and (2) the processing of phonological storage [,]. It could be hypothesized that during this prosodic condition, the participants tended to subvocalize the utterances they heard, and thus reproduce the articulatory gestures necessary for their production. This result can thus be related to the Motor Theory of Speech [], which is similar to that of Hickok and Poeppel [], who propose that the perception of syllables induces the stimulation of the motor activity required for their production. According to the latter authors, phonemes have an audio motor representation rather than a pure auditory representation. More recently, neurobiological theories of speech perception have proposed a more dynamic and integrative model in which language processing relies on action–perception circuits distributed across the auditory and motor systems [,].
Up to now, all the studies we have dealt with concerned the mother tongue (L1), which is why we set up an fMRI study aiming at assessing how the neural processing of second language (L2) comprehension is modulated by the degree of proficiency, making it possible to determine the degree of mastery of the different speech components (prosody, phonology, semantics, and syntax) which are intrinsically embedded within connected speech []. When comparing the neural basis of highly proficient subjects in the second language (L2) with that of moderately proficient subjects, it appears that L2 entails more activation in the bilateral temporal cortex and in the audio-motor loop as well as its right counterpart in highly proficient subjects than in moderately-proficient subjects. It is likely that this finding is due to the difficulties met by moderately proficient subjects in using articulatory-based processes (rehearsal) to keep auditory-based representations (storage) active. Furthermore, all subjects were exposed to an aural dictation of the passage heard in the scanner. This task not only helped determine the global percentage of French students’ general perception of English but also made it possible to classify the percentage of mistakes according to syntactic, phonological, and lexical errors. Interestingly, the correlations between the success scores of the scanning aural dictation and brain activation in Regions of Interest (ROI) known to be involved in phonological, semantics, and complex sentence processes [] revealed significant differences between the 2 groups for phonological and complex sentences processes, but no significant difference was evidenced for semantics, suggesting that semantics is equally acquired for each group, which is in accordance with other studies []. However, activation in a temporal ROI common to phonological, semantics, and complex sentence processes, the left posterior Human Voice Area (pHVA), presented a significant positive correlation with the score of syntax, phonetics, and lexicon. This area, as well as its right counterpart, has been defined as a bilateral region that responds more to human vocal sounds than to environmental sounds [,]. This left pHVA has been described as a “crossroads” region for phonological, semantics, and complex sentence processing [].
Moreover, the right counterpart of the audio-motor loop, was revealed to be involved in prosodic processing such as durational processes or temporal analyses [,] or rhythm analysis tasks [,] was only recruited by highly-proficient subjects, suggesting that moderately-proficient subjects do not process the specific prosodic dimension of the second language. This assumption is supported by the results of a subsequent functional connectivity analysis, which revealed that the left Inferior Frontal Gyrus and its right counterpart were significantly correlated with the left Sylvian parietotemporal (Spt) area in highly proficient subjects, whereas no correlation between these two regions was observed in moderately proficient subjects. The latter result is in accordance with previous work suggesting a functional connection between the auditory cortex and the left prefrontal associative cortex involved both in the retrieval and rehearsal of auditory information and in the auditory working memory in L1 [,]. Moreover, the significant correlation between the right Inferior Frontal Gyrus and the left Spt in highly proficient subjects (and the lack of correlation in moderately proficient subjects) is of considerable interest since it strengthens the idea that moderately proficient subjects miss the prosodic dimension of L2.
All this taken together, this could mean that highly proficient L2 subjects present a mastery of the prosodic dimension which is the basis underlying the linguistic dimension, whereas moderately proficient L2 subjects, missing this prosodic basis, subsequently miss the expression dimension.
At the word level: A few studies have investigated the neural correlates of the production or perception of prosody at the word level. One fMRI study has investigated the existence of right dorsal and ventral processing streams underlying the prosodic dimension which parallel the well-established left ventral and dorsal streams underlying core linguistic abilities such as phonology, syntax, and semantics []. Moreover, the same authors have evidenced that the inhibition of the right premotor cortex decreases participants’ performance in the categorisation of prosody, arguing for motor involvement in the perception of prosody, though no activation was reported in the left premotor cortex or in the inferior frontal gyrus. However, another fMRI study that sought to demonstrate that the perception and production of prosody share common neural areas has revealed that the premotor cortex, including the left inferior frontal gyrus and the left dorsal premotor cortex, was active for both the perception and production of prosody []. This study reveals that some components of the perception of the prosodic dimension involve mapping the heard speech to areas that are important for producing that same speech. Nonetheless, the authors explain their results according to the hypothesis of the “as if body loop” mechanism which postulates that individuals use sensory-motor regions to implicitly simulate perceived or imagined experiences [].
Based on our previous findings concerning the involvement of the prosodic dimension in the recruitment of the phonological loop [], we addressed the question of the hemispheric cooperation, using lists of over-learnt words, known to involve both specific prosodic information, i.e., the metrics of the lists of words, and phonemic information. More particularly, as the audio-motor loop has been revealed to be involved in listening, production, and reading tasks, we inquired both into the existence of core heteromodal areas common to these 3 language modalities and also into the role of the right hemisphere. In order to do so, we examined the Word-List processing areas common to production, listening, and reading tasks obtained with functional Magnetic Resonance Imaging (fMRI) together with a resting-state acquisition in 144 right-handed subjects []. This allowed for the identification of 21 multimodal homotopic regions of interest (hROI) significantly asymmetrical and activated during the 3 task-induced acquisitions. Within these 21 regions, the word-list core network was revealed by a hierarchical cluster analysis based on the resting-state Blood Oxygenation Level Dependent (BOLD) temporal correlations across these 21 hROIs. This supramodal network for word processing aggregates 14 motor, premotor, and phonemic areas in the left hemisphere and the right Superior Temporal Sulcus (STS), which corresponds to the posterior human voice area (pHVA) (Figure 3).
As already discussed, the bilateral pHVA is not only reported to be involved in human voice processing [,] but also in the processing of prosodic acoustic cues, with a rightward involvement concerning the speaker’s identity and gender as well as the affective prosody and a leftward involvement concerning the linguistic prosody []. This functional specialisation is in accordance with studies revealing that the right temporal cortex is involved in spectral processing, i.e. the modulations of the Fundamental Frequency (F0), whereas the left temporal cortex is involved in temporal processes, such as duration and intensity of the utterance [-]. We postulate that the right pHVA is the first prosodic area to be functional, and thanks to the corpus callosum, specific prosodic information is transferred to the left pHVA where it is integrated into phonological-lexical-syntactic processes. These right and left pHVA are probably key areas for communication and language development, supporting the Expression dimension.
Interestingly, the regions belonging to this multimodal network were activated by the 3 modalities, even if they were modulated by the language function: production, reading, or listening. Hence, these 14 regions constitute an atlas of the regions involved in Word-List processing which makes it possible to propose a model of the neural organization of word processing. This model posits that: (1) the involvement of the right STS3 (pHVA), which is a prosodic integrative area, reflects the intertwining between prosodic and phonemic information; (2) the involvement of phonological action-perception circuits, such as the phonological working memory loop, in which articulatory gestures are the central motor units on which word perception, production and reading develop, acts according to the motor theory of speech (Liberman and Whalen, 2000), as revealed by the recruitment of leftward frontal and precentral areas together with temporo-parietal areas.
Considering Fuster’s model, which posits the existence of a perception-action cycle in which functional intracortical connections are bidirectional and link a series of hierarchically organized areas dedicated to cognitive processing in the form of widely distributed neuronal networks (Figure 4), we assume that the model of the word processing network entails reciprocal interactions between the premotor cortex and the unimodal association cortices [,].
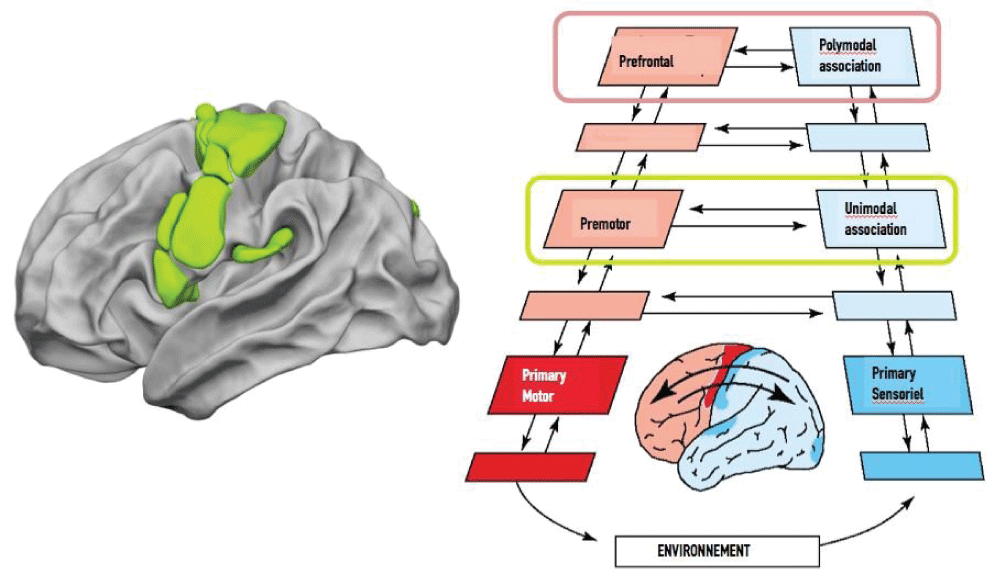 84]." /> Figure 4: The phonological action- perception loop for word processing in relation with Fuster’s model with Fuster’s model [].
84]." /> Figure 4: The phonological action- perception loop for word processing in relation with Fuster’s model with Fuster’s model [].To summarize, observations and behavioural investigations concerning speech perception in foetuses and infants, as well as neuroimaging studies performed on infants and adults favour the theory that the Expressivity dimension, which can be compared to a musical stave, is the basis on which phonemes, syllables, words, grammar and morphosyntax, i.e., the Expression dimension, is superimposed (Figure 2).
Moreover, even if the existence of the phonological action-perception loop, i.e., the reciprocal link between speech perception and production underpinned by the cerebral network made of left temporal and frontal areas (whatever the modality), has been widely established in the neuroimaging literature, we have shown, for the first time, that this phonological loop is triggered by the mastering of the Expressivity dimension, as exemplified by the recruitment of the right Superior Temporal Sulcus (STS3) belonging to the functional network of word processing. This new model, which places the expressivity dimension of speech within the language act itself, is of great interest both in the field of second-language learning and in the rehabilitation of language disorders such as dyslexia or aphasia.
We would like to thank Dr Anne-Marie Carassou and Dr Nathalie Tzourio-Mazoyer for their useful comments.
Memorisation of foreign speech and specific sensory integration of prosody. In from Perception to Understanding a Foreign Language. 2010.
André J, Kelly DO. Systematic Grammar of English (Nathan).
Lacheret-Dujour, Beaugendre. The prosody of French (Paris). 1999.
Nazzi T, Kemler Nelson DG, Jusczyk PW, Jusczyk AM. Six-Month-Olds' Detection of Clauses Embedded in Continuous Speech: Effects of Prosodic Well-Formedness. Infancy. 2000 Jan;1(1):123-147. doi: 10.1207/S15327078IN0101_11. Epub 2000 Jan 1. PMID: 32680315.
Ramus F, Hauser MD, Miller C, Morris D, Mehler J. Language discrimination by human newborns and by cotton-top tamarin monkeys. Science. 2000 Apr 14;288(5464):349-51. doi: 10.1126/science.288.5464.349. PMID: 10764650.
Seidl A. Infants’ use and weighting of prosodic cues in clause segmentation. Journal of Memory and Language. 2007; 57:24–48.
Mehler J, Jusczyk P, Lambertz G, Halsted N, Bertoncini J, Amiel-Tison C. A precursor of language acquisition in young infants. Cognition. 1988 Jul;29(2):143-78. doi: 10.1016/0010-0277(88)90035-2. PMID: 3168420.
Mehler J, Dupoux E, Nazzi T, Dehaene-Lambertz Coping with linguistic diversity: The infant’s viewpoint. In Signal to Syntax: Bootstrapping from Speech to Grammar in Early Acquisition, (Hillsdale, NJ, US: LawrenceErlbaum Associates, Inc). 1996; 101–116.
Interactive developmental intonology: theories and application to French. 177.202.
Snow, Balog. Do children produce the melody before the words? A review of developmental intonation research. 1025–1058.
Pitt MA, Samuel AG. The use of rhythm in attending to speech. J Exp Psychol Hum Percept Perform. 1990 Aug;16(3):564-73. doi: 10.1037//0096-1523.16.3.564. PMID: 2144571.
Schwartze M, Rothermich K, Schmidt-Kassow M, Kotz SA. Temporal regularity effects on pre-attentive and attentive processing of deviance. Biol Psychol. 2011 Apr;87(1):146-51. doi: 10.1016/j.biopsycho.2011.02.021. Epub 2011 Mar 5. PMID: 21382437.
Roncaglia-Denissen MP, Schmidt-Kassow M, Kotz SA. Speech rhythm facilitates syntactic ambiguity resolution: ERP evidence. PLoS One. 2013;8(2):e56000. doi: 10.1371/journal.pone.0056000. Epub 2013 Feb 8. PMID: 23409109; PMCID: PMC3568096.
Accent is predictable (f you are a mind-reader). Language. 1972; 633–644.
Gerhardt KJ, Otto R, Abrams RM, Colle JJ, Burchfield DJ, Peters AJ. Cochlear microphonics recorded from fetal and newborn sheep. Am J Otolaryngol. 1992 Jul-Aug;13(4):226-33. doi: 10.1016/0196-0709(92)90026-p. PMID: 1503196..
Griffiths SK, Brown WS Jr, Gerhardt KJ, Abrams RM, Morris RJ. The perception of speech sounds recorded within the uterus of a pregnant sheep. J Acoust Soc Am. 1994 Oct;96(4):2055-63. doi: 10.1121/1.410147. PMID: 7963021.
Lecanuet JP, Granier-Deferre Speech Stimuli in the Fetal Environment. In Developmental Neurocognition: Speech and Face Processing in the First Year of Life, B de Boysson-Bardies, S de Schonen, P Jusczyk, McNeilage P,Morton J.Dordrecht: 1993; 237–248.
Fifer W, Moon C. The effects of fetal experience with sound. In Fetal Development: A Psychobiological Perspective. Lawrence Erlbaum Associates. 1995; 351–366
Moon C, Cooper RP, Fifer WP. Two-day-olds prefer their native language. Infant Behavior and Development. 1993; 16:495–500.
Spence MJ, DeCasper AJ. Prenatal experience with low-frequency maternal voice sounds influence neonatal perception of maternal voice samples. Infant Behavior and Development. 1987; 10:133–142.
Ockleford EM, Vince MA, Layton C, Reader MR. Responses of neonates to parents' and others' voices. Early Hum Dev. 1988 Nov;18(1):27-36. doi: 10.1016/0378-3782(88)90040-0. PMID: 3234282.
Fernald A, Taeschner T, Dunn J, Papousek M, de Boysson-Bardies B, Fukui I. A cross-language study of prosodic modifications in mothers' and fathers' speech to preverbal infants. J Child Lang. 1989 Oct;16(3):477-501. doi: 10.1017/s0305000900010679. PMID: 2808569.
Moon C, Lagercrantz H, Kuhl PK. Language experienced in utero affects vowel perception after birth: a two-country study. Acta Paediatr. 2013 Feb;102(2):156-60. doi: 10.1111/apa.12098. Epub 2013 Jan 9. PMID: 23173548; PMCID: PMC3543479.
Cheour-Luhtanen M, Alho K, Sainio K, Rinne T, Reinikainen K, Pohjavuori M, Renlund M, Aaltonen O, Eerola O, Näätänen R. The ontogenetically earliest discriminative response of the human brain. Psychophysiology. 1996 Jul;33(4):478-81. doi: 10.1111/j.1469-8986.1996.tb01074.x. PMID: 8753948.
Hepper PG, Shahidullah BS. Development of fetal hearing. Arch Dis Child Fetal Neonatal Ed. 1994 Sep;71(2):F81-7. doi: 10.1136/fn.71.2.f81. PMID: 7979483; PMCID: PMC1061088.
Jardri R, Houfflin-Debarge V, Delion P, Pruvo JP, Thomas P, Pins D. Assessing fetal response to maternal speech using a noninvasive functional brain imaging technique. Int J Dev Neurosci. 2012 Apr;30(2):159-61. doi: 10.1016/j.ijdevneu.2011.11.002. Epub 2011 Nov 23. PMID: 22123457.
May L, Byers-Heinlein K, Gervain J, Werker JF. Language and the newborn brain: does prenatal language experience shape the neonate neural response to speech? Front Psychol. 2011 Sep 21;2:222. doi: 10.3389/fpsyg.2011.00222. PMID: 21960980; PMCID: PMC3177294.
Partanen E, Kujala T, Näätänen R, Liitola A, Sambeth A, Huotilainen M. Learning-induced neural plasticity of speech processing before birth. Proc Natl Acad Sci U S A. 2013 Sep 10;110(37):15145-50. doi: 10.1073/pnas.1302159110. Epub 2013 Aug 26. PMID: 23980148; PMCID: PMC3773755.
Peña M, Maki A, Kovacić D, Dehaene-Lambertz G, Koizumi H, Bouquet F, Mehler J. Sounds and silence: an optical topography study of language recognition at birth. Proc Natl Acad Sci U S A. 2003 Sep 30;100(20):11702-5. doi: 10.1073/pnas.1934290100. Epub 2003 Sep 19. PMID: 14500906; PMCID: PMC208821.
Dehaene-Lambertz G, Dehaene S, Hertz-Pannier L. Functional neuroimaging of speech perception in infants. Science. 2002 Dec 6;298(5600):2013-5. doi: 10.1126/science.1077066. PMID: 12471265.
Sambeth A, Ruohio K, Alku P, Fellman V, Huotilainen M. Sleeping newborns extract prosody from continuous speech. Clin Neurophysiol. 2008 Feb;119(2):332-41. doi: 10.1016/j.clinph.2007.09.144. PMID: 18069059.
Dubois J, Benders M, Lazeyras F, Borradori-Tolsa C, Leuchter RH, Mangin JF, Hüppi PS. Structural asymmetries of perisylvian regions in the preterm newborn. Neuroimage. 2010 Aug 1;52(1):32-42. doi: 10.1016/j.neuroimage.2010.03.054. Epub 2010 Mar 31. PMID: 20362679.
Saito Y, Aoyama S, Kondo T, Fukumoto R, Konishi N, Nakamura K, Kobayashi M, Toshima T. Frontal cerebral blood flow change associated with infant-directed speech. Arch Dis Child Fetal Neonatal Ed. 2007 Mar;92(2):F113-6. doi: 10.1136/adc.2006.097949. Epub 2006 Aug 11. PMID: 16905571; PMCID: PMC2675452.
Floccia C, Nazzi T, Bertoncini J. Unfamiliar voice discrimination for short stimuli in newborns. Developmental Science. 2000; 3:333–343.
Nazzi T, Bertoncini J, Mehler J. Language discrimination by newborns: toward an understanding of the role of rhythm. J Exp Psychol Hum Percept Perform. 1998 Jun;24(3):756-66. doi: 10.1037//0096-1523.24.3.756. PMID: 9627414.
Beckman M, Edwards J. Intonational categories and the articulatory control of duration. In Speech Perception, Production and Linguistic Structure. Tokyo: OHM Publishing Co. 1992; 356–375.
Gout A, Christophe A, Morgan JL. Phonological phrase boundaries constrain lexical access II. Infant data. Journal of Memory and Language. 2004; 51:548–567.
Jusczyk PW. Finding and Remembering Words: Some Beginnings by English-Learning Infants. Current Directions in Psychological Science. 1997; 170–174.
Maye J, Werker JF, Gerken L. Infant sensitivity to distributional information can affect phonetic discrimination. Cognition. 2002 Jan;82(3):B101-11. doi: 10.1016/s0010-0277(01)00157-3. PMID: 11747867.
McMurray B, Aslin RN. Infants are sensitive to within-category variation in speech perception. Cognition. 2005 Mar;95(2):B15-26. doi: 10.1016/j.cognition.2004.07.005. PMID: 15694642.
Millotte S. Le jeune enfant à la découverte des mots. Revue française de linguistique appliquée. 2008; XIII:93–102.
Soderstrom M. The prosodic bootstrapping of phrases: Evidence from prelinguistic infants. Journal of Memory and Language. 2003; 49:249–267.
Jusczyk PW, Hirsh-Pasek K, Nelson DG, Kennedy LJ, Woodward A, Piwoz J. Perception of acoustic correlates of major phrasal units by young infants. Cogn Psychol. 1992 Apr;24(2):252-93. doi: 10.1016/0010-0285(92)90009-q. PMID: 1582173.
Morgan, Demuth. Signal to syntax: An overview. In Signal to Syntax: Bootstrapping from Speech to Grammar in Early Acquisition. Lawrence Erlbaum Associates Inc. 1996; 1-22.
From Simple Input to Complex Grammar. 1986.
Fletcher PC, Henson RN. Frontal lobes and human memory: insights from functional neuroimaging. Brain. 2001 May;124(Pt 5):849-81. doi: 10.1093/brain/124.5.849. PMID: 11335690.
Shallice T, Fletcher P, Frith CD, Grasby P, Frackowiak RS, Dolan RJ. Brain regions associated with acquisition and retrieval of verbal episodic memory. Nature. 1994 Apr 14;368(6472):633-5. doi: 10.1038/368633a0. PMID: 8145849.
Mampe B, Friederici AD, Christophe A, Wermke K. Newborns' cry melody is shaped by their native language. Curr Biol. 2009 Dec 15;19(23):1994-7. doi: 10.1016/j.cub.2009.09.064. Epub 2009 Nov 5. PMID: 19896378.
Nathani S, Ertmer DJ, Stark RE. Assessing vocal development in infants and toddlers. Clin Linguist Phon. 2006 Jul;20(5):351-69. doi: 10.1080/02699200500211451. PMID: 16728333; PMCID: PMC3412408.
The emergence of the sounds of speech in infancy. In Child Phonology, Volume 1, Production (pp. 93–112). New York: Academic Press. 1980.
Beebe B, Alson D, Jaffe J, Feldstein S, Crown C. Vocal congruence in mother-infant play. J Psycholinguist Res. 1988 May;17(3):245-59. doi: 10.1007/BF01686358. PMID: 3411533.
De Boysson-Bardies B, Sagart L, Durand C. Discernible differences in the babbling of infants according to target language. J Child Lang. 1984 Feb;11(1):1-15. doi: 10.1017/s0305000900005559. PMID: 6699104.
Kent RD, Murray AD. Acoustic features of infant vocalic utterances at 3, 6, and 9 months. J Acoust Soc Am. 1982 Aug;72(2):353-65. doi: 10.1121/1.388089. PMID: 7119278.
Vihman MM, Nakai S, DePaolis RA, Hallé P. The role of accentual pattern in early lexical representation. Journal of Memory and Language. 2004; 50:336–353.
Gayraud F, Kern S. Caractéristiques phonologiques des noms en fonction de l’âge d’acquisition. Enfance. 2007; 59:324–338.
Stark RE.Infant vocalization: A comprehensive view. Infant Mental Health Journal. 1981; 2:118–128.
Locke JL.Phonological Acquisition and Change. New York, NY: Academic Press. 1983.
Oller DK, Eilers RE. Similarity of babbling in Spanish- and English-learning babies. J Child Lang. 1982 Oct;9(3):565-77. doi: 10.1017/s0305000900004918. PMID: 7174757.
Stoel-Gammon C. Phonetic inventories, 15-24 months: a longitudinal study. J Speech Hear Res. 1985 Dec;28(4):505-12. doi: 10.1044/jshr.2804.505. PMID: 4087885.
Vihman M, Miller R. Words and babble at the threshold of lexical acquisition. In The Emergent Lexicon (pp. 151–183). New York: Academic Press. 1988.
Vihman MM, Velleman SL. The Construction of a First Phonology. PHO. 2000; 57:255–266.
Chen A, Fikkert P. Intonation of early two-word utterances in Dutch. 2007.
Dore J. Holophrases, speech acts and language universals. Journal of Child Language. 1975; 2:21–40.
Halliday MAK. Learning How to Mean: Explorations in the Development of Language. London: Arnold. 1975.
Snow D. Phrase-final syllable lengthening and intonation in early child speech. J Speech Hear Res. 1994 Aug;37(4):831-40. doi: 10.1044/jshr.3704.831. PMID: 7967570.
Nathani S, Oller DK, Cobo-Lewis AB. Final Syllable Lengthening (FSL) in infant vocalizations. J Child Lang. 2003 Feb;30(1):3-25. doi: 10.1017/s0305000902005433. PMID: 12718291.
Contribution de la prosodie dans la mise en place de la syntaxe chez l’enfant de trois ans. 2008; 81–94.
Rodgon M, Monitz. Single-word usage, cognitive development and the beginnings of combinatorial speech. Cambridge University Press. 1976.
Plaut DC, Kello CT. The emergence of phonology from the interplay of speech comprehension and production: A distributed connectionist approach. In The Emergence of Language. Mahwah, NJ, US: Lawrence Erlbaum Associates Publishers. 1999; 381–415.
Imada T, Zhang Y, Cheour M, Taulu S, Ahonen A, Kuhl PK. Infant speech perception activates Broca's area: a developmental magnetoencephalography study. Neuroreport. 2006 Jul 17;17(10):957-62. doi: 10.1097/01.wnr.0000223387.51704.89. PMID: 16791084.
Kuhl PK, Meltzoff AN. The bimodal perception of speech in infancy. Science. 1982 Dec 10;218(4577):1138-41. doi: 10.1126/science.7146899. PMID: 7146899.
Kuhl PK, Meltzoff AN. Infant vocalizations in response to speech: vocal imitation and developmental change. J Acoust Soc Am. 1996 Oct;100(4 Pt 1):2425-38. doi: 10.1121/1.417951. PMID: 8865648; PMCID: PMC3651031.
Ojanen V, Möttönen R, Pekkola J, Jääskeläinen IP, Joensuu R, Autti T, Sams M. Processing of audiovisual speech in Broca's area. Neuroimage. 2005 Apr 1;25(2):333-8. doi: 10.1016/j.neuroimage.2004.12.001. PMID: 15784412.
Pekkola J, Laasonen M, Ojanen V, Autti T, Jääskeläinen IP, Kujala T, Sams M. Perception of matching and conflicting audiovisual speech in dyslexic and fluent readers: an fMRI study at 3 T. Neuroimage. 2006 Feb 1;29(3):797-807. doi: 10.1016/j.neuroimage.2005.09.069. Epub 2005 Dec 15. PMID: 16359873.
Pulvermüller F, Fadiga L. Active perception: sensorimotor circuits as a cortical basis for language. Nat Rev Neurosci. 2010 May;11(5):351-60. doi: 10.1038/nrn2811. Epub 2010 Apr 9. PMID: 20383203.
Pulvermüller F, Huss M, Kherif F, Moscoso del Prado Martin F, Hauk O, Shtyrov Y. Motor cortex maps articulatory features of speech sounds. Proc Natl Acad Sci U S A. 2006 May 16;103(20):7865-70. doi: 10.1073/pnas.0509989103. Epub 2006 May 8. PMID: 16682637; PMCID: PMC1472536.
Skipper JI, van Wassenhove V, Nusbaum HC, Small SL. Hearing lips and seeing voices: how cortical areas supporting speech production mediate audiovisual speech perception. Cereb Cortex. 2007 Oct;17(10):2387-99. doi: 10.1093/cercor/bhl147. Epub 2007 Jan 11. PMID: 17218482; PMCID: PMC2896890.
Vigneau M, Beaucousin V, Hervé PY, Duffau H, Crivello F, Houdé O, Mazoyer B, Tzourio-Mazoyer N. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. Neuroimage. 2006 May 1;30(4):1414-32. doi: 10.1016/j.neuroimage.2005.11.002. Epub 2006 Jan 18. PMID: 16413796.
Wilson SM, Iacoboni M. Neural responses to non-native phonemes varying in producibility: evidence for the sensorimotor nature of speech perception. Neuroimage. 2006 Oct 15;33(1):316-25. doi: 10.1016/j.neuroimage.2006.05.032. Epub 2006 Aug 17. PMID: 16919478.
Wilson SM, Saygin AP, Sereno MI, Iacoboni M. Listening to speech activates motor areas involved in speech production. Nat Neurosci. 2004 Jul;7(7):701-2. doi: 10.1038/nn1263. Epub 2004 Jun 6. PMID: 15184903.
Liberman AM, Whalen DH. On the relation of speech to language. Trends Cogn Sci. 2000 May;4(5):187-196. doi: 10.1016/s1364-6613(00)01471-6. PMID: 10782105.
Buchsbaum BR, Hickok G, Humphries C. Role of left posterior superior temporal gyrus in phonological processing for speech perception and production. Cognitive Science. 2001; 25:663–678.
Kuhl PK, Ramírez RR, Bosseler A, Lin JF, Imada T. Infants' brain responses to speech suggest analysis by synthesis. Proc Natl Acad Sci U S A. 2014 Aug 5;111(31):11238-45. doi: 10.1073/pnas.1410963111. Epub 2014 Jul 14. PMID: 25024207; PMCID: PMC4128155.
Hesling I, Labache L, Joliot M, Tzourio-Mazoyer N. Large-scale plurimodal networks common to listening to, producing and reading word lists: an fMRI study combining task-induced activation and intrinsic connectivity in 144 right-handers. Brain Struct Funct. 2019 Dec;224(9):3075-3094. doi: 10.1007/s00429-019-01951-4. Epub 2019 Sep 7. PMID: 31494717; PMCID: PMC6875148.
Labache L, Joliot M, Saracco J, Jobard G, Hesling I, Zago L, Mellet E, Petit L, Crivello F, Mazoyer B, Tzourio-Mazoyer N. A SENtence Supramodal Areas AtlaS (SENSAAS) based on multiple task-induced activation mapping and graph analysis of intrinsic connectivity in 144 healthy right-handers. Brain Struct Funct. 2019 Mar;224(2):859-882. doi: 10.1007/s00429-018-1810-2. Epub 2018 Dec 7. PMID: 30535758; PMCID: PMC6420474.
Binder JR, Rao SM, Hammeke TA, Yetkin FZ, Jesmanowicz A, Bandettini PA, Wong EC, Estkowski LD, Goldstein MD, Haughton VM, et al. Functional magnetic resonance imaging of human auditory cortex. Ann Neurol. 1994 Jun;35(6):662-72. doi: 10.1002/ana.410350606. PMID: 8210222.
Caplan D, Alpert N, Waters G. Effects of syntactic structure and propositional number on patterns of regional cerebral blood flow. J Cogn Neurosci. 1998 Jul;10(4):541-52. doi: 10.1162/089892998562843. PMID: 9712683.
Démonet JF, Chollet F, Ramsay S, Cardebat D, Nespoulous JL, Wise R, Rascol A, Frackowiak R. The anatomy of phonological and semantic processing in normal subjects. Brain. 1992 Dec;115 ( Pt 6):1753-68. doi: 10.1093/brain/115.6.1753. PMID: 1486459.
Friederici AD. Towards a neural basis of auditory sentence processing. Trends Cogn Sci. 2002 Feb 1;6(2):78-84. doi: 10.1016/s1364-6613(00)01839-8. PMID: 15866191.
Mazoyer BM, Tzourio N, Frak V, Syrota A, Murayama N, Levrier O, Salamon G, Dehaene S, Cohen L, Mehler J. The cortical representation of speech. J Cogn Neurosci. 1993 Fall;5(4):467-79. doi: 10.1162/jocn.1993.5.4.467. PMID: 23964919.
Barrett AM, Crucian GP, Raymer AM, Heilman KM. Spared comprehension of emotional prosody in a patient with global aphasia. Neuropsychiatry Neuropsychol Behav Neurol. 1999 Apr;12(2):117-20. PMID: 10223259.
Baum SR, Pell MD. The neural bases of prosody: Insights from lesion studies and neuroimaging. Aphasiology. 1999; 13:581–608.
Kawashima R, Itoh M, Hatazawa J, Miyazawa H, Yamada K, Matsuzawa T, Fukuda H. Changes of regional cerebral blood flow during listening to an unfamiliar spoken language. Neurosci Lett. 1993 Oct 14;161(1):69-72. doi: 10.1016/0304-3940(93)90142-8. PMID: 8255550.
Luks TL, Nusbaum HC, Levy J. Hemispheric involvement in the perception of syntactic prosody is dynamically dependent on task demands. Brain Lang. 1998 Nov;65(2):313-32. doi: 10.1006/brln.1998.1993. PMID: 9784273.
Pihan H, Altenmüller E, Hertrich I, Ackermann H. Cortical activation patterns of affective speech processing depend on concurrent demands on the subvocal rehearsal system. A DC-potential study. Brain. 2000 Nov;123 ( Pt 11):2338-49. doi: 10.1093/brain/123.11.2338. PMID: 11050033.
Stirling J, Cavill J, Wilkinson A. Dichotically presented emotionally intoned words produce laterality differences as a function of localisation task. Laterality. 2000 Oct;5(4):363-71. doi: 10.1080/713754388. PMID: 15513153.
Sidtis JJ, Feldmann E. Transient ischemic attacks presenting with a loss of pitch perception. Cortex. 1990 Sep;26(3):469-71. doi: 10.1016/s0010-9452(13)80097-4. PMID: 2249448.
Sidtis JJ, Volpe BT. Selective loss of complex-pitch or speech discrimination after unilateral lesion. Brain Lang. 1988 Jul;34(2):235-45. doi: 10.1016/0093-934x(88)90135-6. PMID: 3401692.
Zatorre RJ. Pitch perception of complex tones and human temporal-lobe function. J Acoust Soc Am. 1988 Aug;84(2):566-72. doi: 10.1121/1.396834. PMID: 3170948.
Blonder LX, Bowers D, Heilman KM. The role of the right hemisphere in emotional communication. Brain. 1991 Jun;114 ( Pt 3):1115-27. doi: 10.1093/brain/114.3.1115. Erratum in: Brain 1992 Apr;115(Pt 2):645. PMID: 2065243.
Grosjean F, Hirt C. Using Prosody to Predict the End of Sentences in English and French: Normal and Brain-Damaged Subjects. Language and Cognitive Processes. 1996; 11:107–134.
Starkstein SE, Federoff JP, Price TR, Leiguarda RC, Robinson RG. Neuropsychological and neuroradiologic correlates of emotional prosody comprehension. Neurology. 1994 Mar;44(3 Pt 1):515-22. doi: 10.1212/wnl.44.3_part_1.515. PMID: 8145924.
Tompkins CA, Flowers CR. Influence of Congruent and Incongruent Contexts on Prosodic Mood Recognition by Brain-Damaged Adults. 1985.
Griffiths TD, Büchel C, Frackowiak RS, Patterson RD. Analysis of temporal structure in sound by the human brain. Nat Neurosci. 1998 Sep;1(5):422-7. doi: 10.1038/1637. PMID: 10196534.
Hesling I, Clément S, Bordessoules M, Allard M. Cerebral mechanisms of prosodic integration: evidence from connected speech. Neuroimage. 2005 Feb 15;24(4):937-47. doi: 10.1016/j.neuroimage.2004.11.003. Epub 2004 Dec 19. PMID: 15670670.
Rauschecker JP, Tian B. Mechanisms and streams for processing of "what" and "where" in auditory cortex. Proc Natl Acad Sci U S A. 2000 Oct 24;97(22):11800-6. doi: 10.1073/pnas.97.22.11800. PMID: 11050212; PMCID: PMC34352.
Weeks RA, Aziz-Sultan A, Bushara KO, Tian B, Wessinger CM, Dang N, Rauschecker JP, Hallett M. A PET study of human auditory spatial processing. Neurosci Lett. 1999 Mar 12;262(3):155-8. doi: 10.1016/s0304-3940(99)00062-2. PMID: 10218879.
Zatorre RJ, Evans AC, Meyer E, Gjedde A. Lateralization of phonetic and pitch discrimination in speech processing. Science. 1992 May 8;256(5058):846-9. doi: 10.1126/science.1589767. PMID: 1589767.
Paulesu E, Frith CD, Frackowiak RS. The neural correlates of the verbal component of working memory. Nature. 1993 Mar 25;362(6418):342-5. doi: 10.1038/362342a0. PMID: 8455719.
Wildgruber D, Kischka U, Ackermann H, Klose U, Grodd W. Dynamic pattern of brain activation during sequencing of word strings evaluated by fMRI. Brain Res Cogn Brain Res. 1999 Jan;7(3):285-94. doi: 10.1016/s0926-6410(98)00031-7. PMID: 9838166.
Hickok G, Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition. 2004 May-Jun;92(1-2):67-99. doi: 10.1016/j.cognition.2003.10.011. PMID: 15037127.
Pulvermüller F, Fadiga L. Chapter 26 - Brain Language Mechanisms Built on Action and Perception. In G. Hickok & S. L. Small (Eds.), Neurobiology of Language. Academic Press. 2016; 311-324.
Hesling I, Dilharreguy B, Bordessoules M, Allard M. The neural processing of second language comprehension modulated by the degree of proficiency: a listening connected speech FMRI study. Open Neuroimag J. 2012;6:44-54. doi: 10.2174/1874440001206010044. Epub 2012 Jul 13. PMID: 22927897; PMCID: PMC3426773.
Wartenburger I, Heekeren HR, Abutalebi J, Cappa SF, Villringer A, Perani D. Early setting of grammatical processing in the bilingual brain. Neuron. 2003 Jan 9;37(1):159-70. doi: 10.1016/s0896-6273(02)01150-9. PMID: 12526781.
Belin P, Zatorre RJ, Lafaille P, Ahad P, Pike B. Voice-selective areas in human auditory cortex. Nature. 2000 Jan 20;403(6767):309-12. doi: 10.1038/35002078. PMID: 10659849.
Kriegstein KV, Giraud AL. Distinct functional substrates along the right superior temporal sulcus for the processing of voices. Neuroimage. 2004 Jun;22(2):948-55. doi: 10.1016/j.neuroimage.2004.02.020. PMID: 15193626.
Fiez JA, Raichle ME, Miezin FM, Petersen SE, Tallal P, Katz WF. PET Studies of Auditory and Phonological Processing: Effects of Stimulus Characteristics and Task Demands. J Cogn Neurosci. 1995 Summer;7(3):357-75. doi: 10.1162/jocn.1995.7.3.357. PMID: 23961866.
Tallal P, Miller S, Fitch RH. Neurobiological basis of speech: A case for the preeminence of temporal processing. In Temporal Information Processing in the Nervous System: Special Reference to Dyslexia and Dysphasia. 1993; 27–47).
Riecker A, Ackermann H, Wildgruber D, Dogil G, Grodd W. Opposite hemispheric lateralization effects during speaking and singing at motor cortex, insula and cerebellum. Neuroreport. 2000 Jun 26;11(9):1997-2000. doi: 10.1097/00001756-200006260-00038. PMID: 10884059.
Schubotz RI, Friederici AD, von Cramon DY. Time perception and motor timing: a common cortical and subcortical basis revealed by fMRI. Neuroimage. 2000 Jan;11(1):1-12. doi: 10.1006/nimg.1999.0514. PMID: 10686112.
Zatorre RJ, Evans AC, Meyer E. Neural mechanisms underlying melodic perception and memory for pitch. J Neurosci. 1994 Apr;14(4):1908-19. doi: 10.1523/JNEUROSCI.14-04-01908.1994. PMID: 8158246; PMCID: PMC6577137.
Zatorre RJ, Halpern AR, Perry DW, Meyer E, Evans AC. Hearing in the Mind's Ear: A PET Investigation of Musical Imagery and Perception. J Cogn Neurosci. 1996 Winter;8(1):29-46. doi: 10.1162/jocn.1996.8.1.29. PMID: 23972234.
Sammler D, Grosbras MH, Anwander A, Bestelmeyer PE, Belin P. Dorsal and Ventral Pathways for Prosody. Curr Biol. 2015 Dec 7;25(23):3079-85. doi: 10.1016/j.cub.2015.10.009. Epub 2015 Nov 5. PMID: 26549262.
Aziz-Zadeh L, Sheng T, Gheytanchi A. Common premotor regions for the perception and production of prosody and correlations with empathy and prosodic ability. PLoS One. 2010 Jan 20;5(1):e8759. doi: 10.1371/journal.pone.0008759. PMID: 20098696; PMCID: PMC2808341.
Damasio AR. The feeling of what happens: Body and emotion in the making of consciousness. New York. 1999.
Belin P, Fecteau S, Bédard C. Thinking the voice: neural correlates of voice perception. Trends Cogn Sci. 2004 Mar;8(3):129-35. doi: 10.1016/j.tics.2004.01.008. PMID: 15301753.
Beaucousin V, Lacheret A, Turbelin MR, Morel M, Mazoyer B, Tzourio-Mazoyer N. FMRI study of emotional speech comprehension. Cereb Cortex. 2007 Feb;17(2):339-52. doi: 10.1093/cercor/bhj151. Epub 2006 Mar 8. PMID: 16525130.
Tervaniemi M, Hugdahl K. Lateralization of auditory-cortex functions. Brain Res Brain Res Rev. 2003 Dec;43(3):231-46. doi: 10.1016/j.brainresrev.2003.08.004. PMID: 14629926.
Zatorre RJ, Belin P. Spectral and temporal processing in human auditory cortex. Cereb Cortex. 2001 Oct;11(10):946-53. doi: 10.1093/cercor/11.10.946. PMID: 11549617.
Zatorre RJ, Belin P, Penhune VB. Structure and function of auditory cortex: music and speech. Trends Cogn Sci (Regul. Ed.). 2002; 6:37–46.
Fuster JM. The prefrontal cortex--an update: time is of the essence. Neuron. 2001 May;30(2):319-33. doi: 10.1016/s0896-6273(01)00285-9. PMID: 11394996.
Fuster JM. The Prefrontal Cortex Makes the Brain a Preadaptive System. Proceedings of the IEEE. 2014; 102:417–426.
Hesling I. The Expressivity Dimension of Speech is the basis of the Expression Dimension. Evidence from Behavioural and Neuroimaging Studies. IgMin Res. May 06, 2024; 2(5): 313-322. IgMin ID: igmin182; DOI: 10.61927/igmin182; Available at: igmin.link/p182
次のリンクを共有した人は、このコンテンツを読むことができます:
1University of Bordeaux, IMN, UMR 5293, 33000 Bordeaux, France
2CNRS, IMN, UMR 5293, 33000 Bordeaux, France
3CEA, GIN, IMN, UMR 5293, 33000 Bordeaux, France
4IMN Institute of Neurodegenerative Diseases UMR 5293, Team 5: GIN Neurofunctional Imaging Group, CEA-CNRS, University of Bordeaux, Center Broca Nouvelle-Aquitaine-3rd fl oor, 146 rue Léo-Saignat-CS 61292-Case 28, 33076 Bordeaux Cedex, France
Address Correspondence:
Isabelle Hesling, University of Bordeaux, IMN, UMR 5293, 33000 Bordeaux, France, Email: [email protected]
How to cite this article:
Hesling I. The Expressivity Dimension of Speech is the basis of the Expression Dimension. Evidence from Behavioural and Neuroimaging Studies. IgMin Res. May 06, 2024; 2(5): 313-322. IgMin ID: igmin182; DOI: 10.61927/igmin182; Available at: igmin.link/p182
Copyright: © 2024 Hesling I. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
![Modality of communication [1,2].](https://www.igminresearch.jp/articles/figures/igmin182/igmin182.g001.png) Figure 1: Modality of communication [1,2]....
Figure 1: Modality of communication [1,2]....
 Figure 2: The Expressivity dimension is the dynamic support ...
Figure 2: The Expressivity dimension is the dynamic support ...
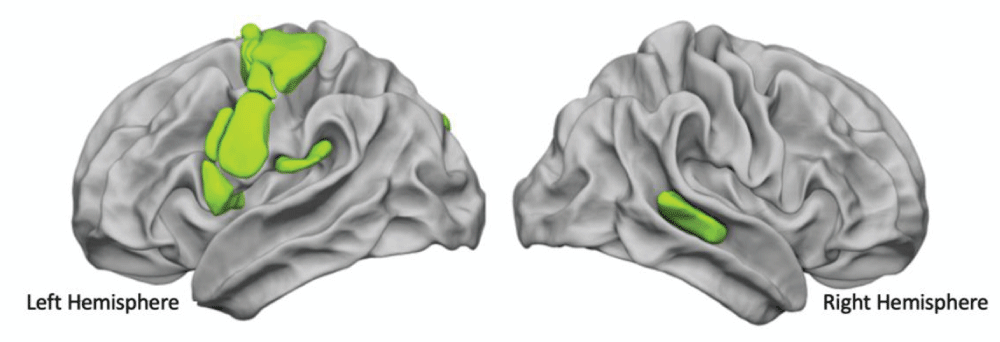 Figure 3: The functional network of word processing common t...
Figure 3: The functional network of word processing common t...
![The phonological action- perception loop for word processing in relation with Fuster’s model with Fuster’s model [84].](https://www.igminresearch.jp/articles/figures/igmin182/igmin182.g004.png) Figure 4: The phonological action- perception loop for word ...
Figure 4: The phonological action- perception loop for word ...
Memorisation of foreign speech and specific sensory integration of prosody. In from Perception to Understanding a Foreign Language. 2010.
André J, Kelly DO. Systematic Grammar of English (Nathan).
Lacheret-Dujour, Beaugendre. The prosody of French (Paris). 1999.
Nazzi T, Kemler Nelson DG, Jusczyk PW, Jusczyk AM. Six-Month-Olds' Detection of Clauses Embedded in Continuous Speech: Effects of Prosodic Well-Formedness. Infancy. 2000 Jan;1(1):123-147. doi: 10.1207/S15327078IN0101_11. Epub 2000 Jan 1. PMID: 32680315.
Ramus F, Hauser MD, Miller C, Morris D, Mehler J. Language discrimination by human newborns and by cotton-top tamarin monkeys. Science. 2000 Apr 14;288(5464):349-51. doi: 10.1126/science.288.5464.349. PMID: 10764650.
Seidl A. Infants’ use and weighting of prosodic cues in clause segmentation. Journal of Memory and Language. 2007; 57:24–48.
Mehler J, Jusczyk P, Lambertz G, Halsted N, Bertoncini J, Amiel-Tison C. A precursor of language acquisition in young infants. Cognition. 1988 Jul;29(2):143-78. doi: 10.1016/0010-0277(88)90035-2. PMID: 3168420.
Mehler J, Dupoux E, Nazzi T, Dehaene-Lambertz Coping with linguistic diversity: The infant’s viewpoint. In Signal to Syntax: Bootstrapping from Speech to Grammar in Early Acquisition, (Hillsdale, NJ, US: LawrenceErlbaum Associates, Inc). 1996; 101–116.
Interactive developmental intonology: theories and application to French. 177.202.
Snow, Balog. Do children produce the melody before the words? A review of developmental intonation research. 1025–1058.
Pitt MA, Samuel AG. The use of rhythm in attending to speech. J Exp Psychol Hum Percept Perform. 1990 Aug;16(3):564-73. doi: 10.1037//0096-1523.16.3.564. PMID: 2144571.
Schwartze M, Rothermich K, Schmidt-Kassow M, Kotz SA. Temporal regularity effects on pre-attentive and attentive processing of deviance. Biol Psychol. 2011 Apr;87(1):146-51. doi: 10.1016/j.biopsycho.2011.02.021. Epub 2011 Mar 5. PMID: 21382437.
Roncaglia-Denissen MP, Schmidt-Kassow M, Kotz SA. Speech rhythm facilitates syntactic ambiguity resolution: ERP evidence. PLoS One. 2013;8(2):e56000. doi: 10.1371/journal.pone.0056000. Epub 2013 Feb 8. PMID: 23409109; PMCID: PMC3568096.
Accent is predictable (f you are a mind-reader). Language. 1972; 633–644.
Gerhardt KJ, Otto R, Abrams RM, Colle JJ, Burchfield DJ, Peters AJ. Cochlear microphonics recorded from fetal and newborn sheep. Am J Otolaryngol. 1992 Jul-Aug;13(4):226-33. doi: 10.1016/0196-0709(92)90026-p. PMID: 1503196..
Griffiths SK, Brown WS Jr, Gerhardt KJ, Abrams RM, Morris RJ. The perception of speech sounds recorded within the uterus of a pregnant sheep. J Acoust Soc Am. 1994 Oct;96(4):2055-63. doi: 10.1121/1.410147. PMID: 7963021.
Lecanuet JP, Granier-Deferre Speech Stimuli in the Fetal Environment. In Developmental Neurocognition: Speech and Face Processing in the First Year of Life, B de Boysson-Bardies, S de Schonen, P Jusczyk, McNeilage P,Morton J.Dordrecht: 1993; 237–248.
Fifer W, Moon C. The effects of fetal experience with sound. In Fetal Development: A Psychobiological Perspective. Lawrence Erlbaum Associates. 1995; 351–366
Moon C, Cooper RP, Fifer WP. Two-day-olds prefer their native language. Infant Behavior and Development. 1993; 16:495–500.
Spence MJ, DeCasper AJ. Prenatal experience with low-frequency maternal voice sounds influence neonatal perception of maternal voice samples. Infant Behavior and Development. 1987; 10:133–142.
Ockleford EM, Vince MA, Layton C, Reader MR. Responses of neonates to parents' and others' voices. Early Hum Dev. 1988 Nov;18(1):27-36. doi: 10.1016/0378-3782(88)90040-0. PMID: 3234282.
Fernald A, Taeschner T, Dunn J, Papousek M, de Boysson-Bardies B, Fukui I. A cross-language study of prosodic modifications in mothers' and fathers' speech to preverbal infants. J Child Lang. 1989 Oct;16(3):477-501. doi: 10.1017/s0305000900010679. PMID: 2808569.
Moon C, Lagercrantz H, Kuhl PK. Language experienced in utero affects vowel perception after birth: a two-country study. Acta Paediatr. 2013 Feb;102(2):156-60. doi: 10.1111/apa.12098. Epub 2013 Jan 9. PMID: 23173548; PMCID: PMC3543479.
Cheour-Luhtanen M, Alho K, Sainio K, Rinne T, Reinikainen K, Pohjavuori M, Renlund M, Aaltonen O, Eerola O, Näätänen R. The ontogenetically earliest discriminative response of the human brain. Psychophysiology. 1996 Jul;33(4):478-81. doi: 10.1111/j.1469-8986.1996.tb01074.x. PMID: 8753948.
Hepper PG, Shahidullah BS. Development of fetal hearing. Arch Dis Child Fetal Neonatal Ed. 1994 Sep;71(2):F81-7. doi: 10.1136/fn.71.2.f81. PMID: 7979483; PMCID: PMC1061088.
Jardri R, Houfflin-Debarge V, Delion P, Pruvo JP, Thomas P, Pins D. Assessing fetal response to maternal speech using a noninvasive functional brain imaging technique. Int J Dev Neurosci. 2012 Apr;30(2):159-61. doi: 10.1016/j.ijdevneu.2011.11.002. Epub 2011 Nov 23. PMID: 22123457.
May L, Byers-Heinlein K, Gervain J, Werker JF. Language and the newborn brain: does prenatal language experience shape the neonate neural response to speech? Front Psychol. 2011 Sep 21;2:222. doi: 10.3389/fpsyg.2011.00222. PMID: 21960980; PMCID: PMC3177294.
Partanen E, Kujala T, Näätänen R, Liitola A, Sambeth A, Huotilainen M. Learning-induced neural plasticity of speech processing before birth. Proc Natl Acad Sci U S A. 2013 Sep 10;110(37):15145-50. doi: 10.1073/pnas.1302159110. Epub 2013 Aug 26. PMID: 23980148; PMCID: PMC3773755.
Peña M, Maki A, Kovacić D, Dehaene-Lambertz G, Koizumi H, Bouquet F, Mehler J. Sounds and silence: an optical topography study of language recognition at birth. Proc Natl Acad Sci U S A. 2003 Sep 30;100(20):11702-5. doi: 10.1073/pnas.1934290100. Epub 2003 Sep 19. PMID: 14500906; PMCID: PMC208821.
Dehaene-Lambertz G, Dehaene S, Hertz-Pannier L. Functional neuroimaging of speech perception in infants. Science. 2002 Dec 6;298(5600):2013-5. doi: 10.1126/science.1077066. PMID: 12471265.
Sambeth A, Ruohio K, Alku P, Fellman V, Huotilainen M. Sleeping newborns extract prosody from continuous speech. Clin Neurophysiol. 2008 Feb;119(2):332-41. doi: 10.1016/j.clinph.2007.09.144. PMID: 18069059.
Dubois J, Benders M, Lazeyras F, Borradori-Tolsa C, Leuchter RH, Mangin JF, Hüppi PS. Structural asymmetries of perisylvian regions in the preterm newborn. Neuroimage. 2010 Aug 1;52(1):32-42. doi: 10.1016/j.neuroimage.2010.03.054. Epub 2010 Mar 31. PMID: 20362679.
Saito Y, Aoyama S, Kondo T, Fukumoto R, Konishi N, Nakamura K, Kobayashi M, Toshima T. Frontal cerebral blood flow change associated with infant-directed speech. Arch Dis Child Fetal Neonatal Ed. 2007 Mar;92(2):F113-6. doi: 10.1136/adc.2006.097949. Epub 2006 Aug 11. PMID: 16905571; PMCID: PMC2675452.
Floccia C, Nazzi T, Bertoncini J. Unfamiliar voice discrimination for short stimuli in newborns. Developmental Science. 2000; 3:333–343.
Nazzi T, Bertoncini J, Mehler J. Language discrimination by newborns: toward an understanding of the role of rhythm. J Exp Psychol Hum Percept Perform. 1998 Jun;24(3):756-66. doi: 10.1037//0096-1523.24.3.756. PMID: 9627414.
Beckman M, Edwards J. Intonational categories and the articulatory control of duration. In Speech Perception, Production and Linguistic Structure. Tokyo: OHM Publishing Co. 1992; 356–375.
Gout A, Christophe A, Morgan JL. Phonological phrase boundaries constrain lexical access II. Infant data. Journal of Memory and Language. 2004; 51:548–567.
Jusczyk PW. Finding and Remembering Words: Some Beginnings by English-Learning Infants. Current Directions in Psychological Science. 1997; 170–174.
Maye J, Werker JF, Gerken L. Infant sensitivity to distributional information can affect phonetic discrimination. Cognition. 2002 Jan;82(3):B101-11. doi: 10.1016/s0010-0277(01)00157-3. PMID: 11747867.
McMurray B, Aslin RN. Infants are sensitive to within-category variation in speech perception. Cognition. 2005 Mar;95(2):B15-26. doi: 10.1016/j.cognition.2004.07.005. PMID: 15694642.
Millotte S. Le jeune enfant à la découverte des mots. Revue française de linguistique appliquée. 2008; XIII:93–102.
Soderstrom M. The prosodic bootstrapping of phrases: Evidence from prelinguistic infants. Journal of Memory and Language. 2003; 49:249–267.
Jusczyk PW, Hirsh-Pasek K, Nelson DG, Kennedy LJ, Woodward A, Piwoz J. Perception of acoustic correlates of major phrasal units by young infants. Cogn Psychol. 1992 Apr;24(2):252-93. doi: 10.1016/0010-0285(92)90009-q. PMID: 1582173.
Morgan, Demuth. Signal to syntax: An overview. In Signal to Syntax: Bootstrapping from Speech to Grammar in Early Acquisition. Lawrence Erlbaum Associates Inc. 1996; 1-22.
From Simple Input to Complex Grammar. 1986.
Fletcher PC, Henson RN. Frontal lobes and human memory: insights from functional neuroimaging. Brain. 2001 May;124(Pt 5):849-81. doi: 10.1093/brain/124.5.849. PMID: 11335690.
Shallice T, Fletcher P, Frith CD, Grasby P, Frackowiak RS, Dolan RJ. Brain regions associated with acquisition and retrieval of verbal episodic memory. Nature. 1994 Apr 14;368(6472):633-5. doi: 10.1038/368633a0. PMID: 8145849.
Mampe B, Friederici AD, Christophe A, Wermke K. Newborns' cry melody is shaped by their native language. Curr Biol. 2009 Dec 15;19(23):1994-7. doi: 10.1016/j.cub.2009.09.064. Epub 2009 Nov 5. PMID: 19896378.
Nathani S, Ertmer DJ, Stark RE. Assessing vocal development in infants and toddlers. Clin Linguist Phon. 2006 Jul;20(5):351-69. doi: 10.1080/02699200500211451. PMID: 16728333; PMCID: PMC3412408.
The emergence of the sounds of speech in infancy. In Child Phonology, Volume 1, Production (pp. 93–112). New York: Academic Press. 1980.
Beebe B, Alson D, Jaffe J, Feldstein S, Crown C. Vocal congruence in mother-infant play. J Psycholinguist Res. 1988 May;17(3):245-59. doi: 10.1007/BF01686358. PMID: 3411533.
De Boysson-Bardies B, Sagart L, Durand C. Discernible differences in the babbling of infants according to target language. J Child Lang. 1984 Feb;11(1):1-15. doi: 10.1017/s0305000900005559. PMID: 6699104.
Kent RD, Murray AD. Acoustic features of infant vocalic utterances at 3, 6, and 9 months. J Acoust Soc Am. 1982 Aug;72(2):353-65. doi: 10.1121/1.388089. PMID: 7119278.
Vihman MM, Nakai S, DePaolis RA, Hallé P. The role of accentual pattern in early lexical representation. Journal of Memory and Language. 2004; 50:336–353.
Gayraud F, Kern S. Caractéristiques phonologiques des noms en fonction de l’âge d’acquisition. Enfance. 2007; 59:324–338.
Stark RE.Infant vocalization: A comprehensive view. Infant Mental Health Journal. 1981; 2:118–128.
Locke JL.Phonological Acquisition and Change. New York, NY: Academic Press. 1983.
Oller DK, Eilers RE. Similarity of babbling in Spanish- and English-learning babies. J Child Lang. 1982 Oct;9(3):565-77. doi: 10.1017/s0305000900004918. PMID: 7174757.
Stoel-Gammon C. Phonetic inventories, 15-24 months: a longitudinal study. J Speech Hear Res. 1985 Dec;28(4):505-12. doi: 10.1044/jshr.2804.505. PMID: 4087885.
Vihman M, Miller R. Words and babble at the threshold of lexical acquisition. In The Emergent Lexicon (pp. 151–183). New York: Academic Press. 1988.
Vihman MM, Velleman SL. The Construction of a First Phonology. PHO. 2000; 57:255–266.
Chen A, Fikkert P. Intonation of early two-word utterances in Dutch. 2007.
Dore J. Holophrases, speech acts and language universals. Journal of Child Language. 1975; 2:21–40.
Halliday MAK. Learning How to Mean: Explorations in the Development of Language. London: Arnold. 1975.
Snow D. Phrase-final syllable lengthening and intonation in early child speech. J Speech Hear Res. 1994 Aug;37(4):831-40. doi: 10.1044/jshr.3704.831. PMID: 7967570.
Nathani S, Oller DK, Cobo-Lewis AB. Final Syllable Lengthening (FSL) in infant vocalizations. J Child Lang. 2003 Feb;30(1):3-25. doi: 10.1017/s0305000902005433. PMID: 12718291.
Contribution de la prosodie dans la mise en place de la syntaxe chez l’enfant de trois ans. 2008; 81–94.
Rodgon M, Monitz. Single-word usage, cognitive development and the beginnings of combinatorial speech. Cambridge University Press. 1976.
Plaut DC, Kello CT. The emergence of phonology from the interplay of speech comprehension and production: A distributed connectionist approach. In The Emergence of Language. Mahwah, NJ, US: Lawrence Erlbaum Associates Publishers. 1999; 381–415.
Imada T, Zhang Y, Cheour M, Taulu S, Ahonen A, Kuhl PK. Infant speech perception activates Broca's area: a developmental magnetoencephalography study. Neuroreport. 2006 Jul 17;17(10):957-62. doi: 10.1097/01.wnr.0000223387.51704.89. PMID: 16791084.
Kuhl PK, Meltzoff AN. The bimodal perception of speech in infancy. Science. 1982 Dec 10;218(4577):1138-41. doi: 10.1126/science.7146899. PMID: 7146899.
Kuhl PK, Meltzoff AN. Infant vocalizations in response to speech: vocal imitation and developmental change. J Acoust Soc Am. 1996 Oct;100(4 Pt 1):2425-38. doi: 10.1121/1.417951. PMID: 8865648; PMCID: PMC3651031.
Ojanen V, Möttönen R, Pekkola J, Jääskeläinen IP, Joensuu R, Autti T, Sams M. Processing of audiovisual speech in Broca's area. Neuroimage. 2005 Apr 1;25(2):333-8. doi: 10.1016/j.neuroimage.2004.12.001. PMID: 15784412.
Pekkola J, Laasonen M, Ojanen V, Autti T, Jääskeläinen IP, Kujala T, Sams M. Perception of matching and conflicting audiovisual speech in dyslexic and fluent readers: an fMRI study at 3 T. Neuroimage. 2006 Feb 1;29(3):797-807. doi: 10.1016/j.neuroimage.2005.09.069. Epub 2005 Dec 15. PMID: 16359873.
Pulvermüller F, Fadiga L. Active perception: sensorimotor circuits as a cortical basis for language. Nat Rev Neurosci. 2010 May;11(5):351-60. doi: 10.1038/nrn2811. Epub 2010 Apr 9. PMID: 20383203.
Pulvermüller F, Huss M, Kherif F, Moscoso del Prado Martin F, Hauk O, Shtyrov Y. Motor cortex maps articulatory features of speech sounds. Proc Natl Acad Sci U S A. 2006 May 16;103(20):7865-70. doi: 10.1073/pnas.0509989103. Epub 2006 May 8. PMID: 16682637; PMCID: PMC1472536.
Skipper JI, van Wassenhove V, Nusbaum HC, Small SL. Hearing lips and seeing voices: how cortical areas supporting speech production mediate audiovisual speech perception. Cereb Cortex. 2007 Oct;17(10):2387-99. doi: 10.1093/cercor/bhl147. Epub 2007 Jan 11. PMID: 17218482; PMCID: PMC2896890.
Vigneau M, Beaucousin V, Hervé PY, Duffau H, Crivello F, Houdé O, Mazoyer B, Tzourio-Mazoyer N. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. Neuroimage. 2006 May 1;30(4):1414-32. doi: 10.1016/j.neuroimage.2005.11.002. Epub 2006 Jan 18. PMID: 16413796.
Wilson SM, Iacoboni M. Neural responses to non-native phonemes varying in producibility: evidence for the sensorimotor nature of speech perception. Neuroimage. 2006 Oct 15;33(1):316-25. doi: 10.1016/j.neuroimage.2006.05.032. Epub 2006 Aug 17. PMID: 16919478.
Wilson SM, Saygin AP, Sereno MI, Iacoboni M. Listening to speech activates motor areas involved in speech production. Nat Neurosci. 2004 Jul;7(7):701-2. doi: 10.1038/nn1263. Epub 2004 Jun 6. PMID: 15184903.
Liberman AM, Whalen DH. On the relation of speech to language. Trends Cogn Sci. 2000 May;4(5):187-196. doi: 10.1016/s1364-6613(00)01471-6. PMID: 10782105.
Buchsbaum BR, Hickok G, Humphries C. Role of left posterior superior temporal gyrus in phonological processing for speech perception and production. Cognitive Science. 2001; 25:663–678.
Kuhl PK, Ramírez RR, Bosseler A, Lin JF, Imada T. Infants' brain responses to speech suggest analysis by synthesis. Proc Natl Acad Sci U S A. 2014 Aug 5;111(31):11238-45. doi: 10.1073/pnas.1410963111. Epub 2014 Jul 14. PMID: 25024207; PMCID: PMC4128155.
Hesling I, Labache L, Joliot M, Tzourio-Mazoyer N. Large-scale plurimodal networks common to listening to, producing and reading word lists: an fMRI study combining task-induced activation and intrinsic connectivity in 144 right-handers. Brain Struct Funct. 2019 Dec;224(9):3075-3094. doi: 10.1007/s00429-019-01951-4. Epub 2019 Sep 7. PMID: 31494717; PMCID: PMC6875148.
Labache L, Joliot M, Saracco J, Jobard G, Hesling I, Zago L, Mellet E, Petit L, Crivello F, Mazoyer B, Tzourio-Mazoyer N. A SENtence Supramodal Areas AtlaS (SENSAAS) based on multiple task-induced activation mapping and graph analysis of intrinsic connectivity in 144 healthy right-handers. Brain Struct Funct. 2019 Mar;224(2):859-882. doi: 10.1007/s00429-018-1810-2. Epub 2018 Dec 7. PMID: 30535758; PMCID: PMC6420474.
Binder JR, Rao SM, Hammeke TA, Yetkin FZ, Jesmanowicz A, Bandettini PA, Wong EC, Estkowski LD, Goldstein MD, Haughton VM, et al. Functional magnetic resonance imaging of human auditory cortex. Ann Neurol. 1994 Jun;35(6):662-72. doi: 10.1002/ana.410350606. PMID: 8210222.
Caplan D, Alpert N, Waters G. Effects of syntactic structure and propositional number on patterns of regional cerebral blood flow. J Cogn Neurosci. 1998 Jul;10(4):541-52. doi: 10.1162/089892998562843. PMID: 9712683.
Démonet JF, Chollet F, Ramsay S, Cardebat D, Nespoulous JL, Wise R, Rascol A, Frackowiak R. The anatomy of phonological and semantic processing in normal subjects. Brain. 1992 Dec;115 ( Pt 6):1753-68. doi: 10.1093/brain/115.6.1753. PMID: 1486459.
Friederici AD. Towards a neural basis of auditory sentence processing. Trends Cogn Sci. 2002 Feb 1;6(2):78-84. doi: 10.1016/s1364-6613(00)01839-8. PMID: 15866191.
Mazoyer BM, Tzourio N, Frak V, Syrota A, Murayama N, Levrier O, Salamon G, Dehaene S, Cohen L, Mehler J. The cortical representation of speech. J Cogn Neurosci. 1993 Fall;5(4):467-79. doi: 10.1162/jocn.1993.5.4.467. PMID: 23964919.
Barrett AM, Crucian GP, Raymer AM, Heilman KM. Spared comprehension of emotional prosody in a patient with global aphasia. Neuropsychiatry Neuropsychol Behav Neurol. 1999 Apr;12(2):117-20. PMID: 10223259.
Baum SR, Pell MD. The neural bases of prosody: Insights from lesion studies and neuroimaging. Aphasiology. 1999; 13:581–608.
Kawashima R, Itoh M, Hatazawa J, Miyazawa H, Yamada K, Matsuzawa T, Fukuda H. Changes of regional cerebral blood flow during listening to an unfamiliar spoken language. Neurosci Lett. 1993 Oct 14;161(1):69-72. doi: 10.1016/0304-3940(93)90142-8. PMID: 8255550.
Luks TL, Nusbaum HC, Levy J. Hemispheric involvement in the perception of syntactic prosody is dynamically dependent on task demands. Brain Lang. 1998 Nov;65(2):313-32. doi: 10.1006/brln.1998.1993. PMID: 9784273.
Pihan H, Altenmüller E, Hertrich I, Ackermann H. Cortical activation patterns of affective speech processing depend on concurrent demands on the subvocal rehearsal system. A DC-potential study. Brain. 2000 Nov;123 ( Pt 11):2338-49. doi: 10.1093/brain/123.11.2338. PMID: 11050033.
Stirling J, Cavill J, Wilkinson A. Dichotically presented emotionally intoned words produce laterality differences as a function of localisation task. Laterality. 2000 Oct;5(4):363-71. doi: 10.1080/713754388. PMID: 15513153.
Sidtis JJ, Feldmann E. Transient ischemic attacks presenting with a loss of pitch perception. Cortex. 1990 Sep;26(3):469-71. doi: 10.1016/s0010-9452(13)80097-4. PMID: 2249448.
Sidtis JJ, Volpe BT. Selective loss of complex-pitch or speech discrimination after unilateral lesion. Brain Lang. 1988 Jul;34(2):235-45. doi: 10.1016/0093-934x(88)90135-6. PMID: 3401692.
Zatorre RJ. Pitch perception of complex tones and human temporal-lobe function. J Acoust Soc Am. 1988 Aug;84(2):566-72. doi: 10.1121/1.396834. PMID: 3170948.
Blonder LX, Bowers D, Heilman KM. The role of the right hemisphere in emotional communication. Brain. 1991 Jun;114 ( Pt 3):1115-27. doi: 10.1093/brain/114.3.1115. Erratum in: Brain 1992 Apr;115(Pt 2):645. PMID: 2065243.
Grosjean F, Hirt C. Using Prosody to Predict the End of Sentences in English and French: Normal and Brain-Damaged Subjects. Language and Cognitive Processes. 1996; 11:107–134.
Starkstein SE, Federoff JP, Price TR, Leiguarda RC, Robinson RG. Neuropsychological and neuroradiologic correlates of emotional prosody comprehension. Neurology. 1994 Mar;44(3 Pt 1):515-22. doi: 10.1212/wnl.44.3_part_1.515. PMID: 8145924.
Tompkins CA, Flowers CR. Influence of Congruent and Incongruent Contexts on Prosodic Mood Recognition by Brain-Damaged Adults. 1985.
Griffiths TD, Büchel C, Frackowiak RS, Patterson RD. Analysis of temporal structure in sound by the human brain. Nat Neurosci. 1998 Sep;1(5):422-7. doi: 10.1038/1637. PMID: 10196534.
Hesling I, Clément S, Bordessoules M, Allard M. Cerebral mechanisms of prosodic integration: evidence from connected speech. Neuroimage. 2005 Feb 15;24(4):937-47. doi: 10.1016/j.neuroimage.2004.11.003. Epub 2004 Dec 19. PMID: 15670670.
Rauschecker JP, Tian B. Mechanisms and streams for processing of "what" and "where" in auditory cortex. Proc Natl Acad Sci U S A. 2000 Oct 24;97(22):11800-6. doi: 10.1073/pnas.97.22.11800. PMID: 11050212; PMCID: PMC34352.
Weeks RA, Aziz-Sultan A, Bushara KO, Tian B, Wessinger CM, Dang N, Rauschecker JP, Hallett M. A PET study of human auditory spatial processing. Neurosci Lett. 1999 Mar 12;262(3):155-8. doi: 10.1016/s0304-3940(99)00062-2. PMID: 10218879.
Zatorre RJ, Evans AC, Meyer E, Gjedde A. Lateralization of phonetic and pitch discrimination in speech processing. Science. 1992 May 8;256(5058):846-9. doi: 10.1126/science.1589767. PMID: 1589767.
Paulesu E, Frith CD, Frackowiak RS. The neural correlates of the verbal component of working memory. Nature. 1993 Mar 25;362(6418):342-5. doi: 10.1038/362342a0. PMID: 8455719.
Wildgruber D, Kischka U, Ackermann H, Klose U, Grodd W. Dynamic pattern of brain activation during sequencing of word strings evaluated by fMRI. Brain Res Cogn Brain Res. 1999 Jan;7(3):285-94. doi: 10.1016/s0926-6410(98)00031-7. PMID: 9838166.
Hickok G, Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition. 2004 May-Jun;92(1-2):67-99. doi: 10.1016/j.cognition.2003.10.011. PMID: 15037127.
Pulvermüller F, Fadiga L. Chapter 26 - Brain Language Mechanisms Built on Action and Perception. In G. Hickok & S. L. Small (Eds.), Neurobiology of Language. Academic Press. 2016; 311-324.
Hesling I, Dilharreguy B, Bordessoules M, Allard M. The neural processing of second language comprehension modulated by the degree of proficiency: a listening connected speech FMRI study. Open Neuroimag J. 2012;6:44-54. doi: 10.2174/1874440001206010044. Epub 2012 Jul 13. PMID: 22927897; PMCID: PMC3426773.
Wartenburger I, Heekeren HR, Abutalebi J, Cappa SF, Villringer A, Perani D. Early setting of grammatical processing in the bilingual brain. Neuron. 2003 Jan 9;37(1):159-70. doi: 10.1016/s0896-6273(02)01150-9. PMID: 12526781.
Belin P, Zatorre RJ, Lafaille P, Ahad P, Pike B. Voice-selective areas in human auditory cortex. Nature. 2000 Jan 20;403(6767):309-12. doi: 10.1038/35002078. PMID: 10659849.
Kriegstein KV, Giraud AL. Distinct functional substrates along the right superior temporal sulcus for the processing of voices. Neuroimage. 2004 Jun;22(2):948-55. doi: 10.1016/j.neuroimage.2004.02.020. PMID: 15193626.
Fiez JA, Raichle ME, Miezin FM, Petersen SE, Tallal P, Katz WF. PET Studies of Auditory and Phonological Processing: Effects of Stimulus Characteristics and Task Demands. J Cogn Neurosci. 1995 Summer;7(3):357-75. doi: 10.1162/jocn.1995.7.3.357. PMID: 23961866.
Tallal P, Miller S, Fitch RH. Neurobiological basis of speech: A case for the preeminence of temporal processing. In Temporal Information Processing in the Nervous System: Special Reference to Dyslexia and Dysphasia. 1993; 27–47).
Riecker A, Ackermann H, Wildgruber D, Dogil G, Grodd W. Opposite hemispheric lateralization effects during speaking and singing at motor cortex, insula and cerebellum. Neuroreport. 2000 Jun 26;11(9):1997-2000. doi: 10.1097/00001756-200006260-00038. PMID: 10884059.
Schubotz RI, Friederici AD, von Cramon DY. Time perception and motor timing: a common cortical and subcortical basis revealed by fMRI. Neuroimage. 2000 Jan;11(1):1-12. doi: 10.1006/nimg.1999.0514. PMID: 10686112.
Zatorre RJ, Evans AC, Meyer E. Neural mechanisms underlying melodic perception and memory for pitch. J Neurosci. 1994 Apr;14(4):1908-19. doi: 10.1523/JNEUROSCI.14-04-01908.1994. PMID: 8158246; PMCID: PMC6577137.
Zatorre RJ, Halpern AR, Perry DW, Meyer E, Evans AC. Hearing in the Mind's Ear: A PET Investigation of Musical Imagery and Perception. J Cogn Neurosci. 1996 Winter;8(1):29-46. doi: 10.1162/jocn.1996.8.1.29. PMID: 23972234.
Sammler D, Grosbras MH, Anwander A, Bestelmeyer PE, Belin P. Dorsal and Ventral Pathways for Prosody. Curr Biol. 2015 Dec 7;25(23):3079-85. doi: 10.1016/j.cub.2015.10.009. Epub 2015 Nov 5. PMID: 26549262.
Aziz-Zadeh L, Sheng T, Gheytanchi A. Common premotor regions for the perception and production of prosody and correlations with empathy and prosodic ability. PLoS One. 2010 Jan 20;5(1):e8759. doi: 10.1371/journal.pone.0008759. PMID: 20098696; PMCID: PMC2808341.
Damasio AR. The feeling of what happens: Body and emotion in the making of consciousness. New York. 1999.
Belin P, Fecteau S, Bédard C. Thinking the voice: neural correlates of voice perception. Trends Cogn Sci. 2004 Mar;8(3):129-35. doi: 10.1016/j.tics.2004.01.008. PMID: 15301753.
Beaucousin V, Lacheret A, Turbelin MR, Morel M, Mazoyer B, Tzourio-Mazoyer N. FMRI study of emotional speech comprehension. Cereb Cortex. 2007 Feb;17(2):339-52. doi: 10.1093/cercor/bhj151. Epub 2006 Mar 8. PMID: 16525130.
Tervaniemi M, Hugdahl K. Lateralization of auditory-cortex functions. Brain Res Brain Res Rev. 2003 Dec;43(3):231-46. doi: 10.1016/j.brainresrev.2003.08.004. PMID: 14629926.
Zatorre RJ, Belin P. Spectral and temporal processing in human auditory cortex. Cereb Cortex. 2001 Oct;11(10):946-53. doi: 10.1093/cercor/11.10.946. PMID: 11549617.
Zatorre RJ, Belin P, Penhune VB. Structure and function of auditory cortex: music and speech. Trends Cogn Sci (Regul. Ed.). 2002; 6:37–46.
Fuster JM. The prefrontal cortex--an update: time is of the essence. Neuron. 2001 May;30(2):319-33. doi: 10.1016/s0896-6273(01)00285-9. PMID: 11394996.
Fuster JM. The Prefrontal Cortex Makes the Brain a Preadaptive System. Proceedings of the IEEE. 2014; 102:417–426.