
Breast Cancer: The Road to a Personalized Prevention
Oncology Radiation Therapy受け取った 12 Jan 2024 受け入れられた 25 Mar 2024 オンラインで公開された 26 Mar 2024
ISSN: 2995-8067 | Quick Google Scholar
Next Full Text
Preventing Rectal Toxicity in Prostate Cancer: Diet and Supplement Alternative to Enemas or Rectal Spacer


受け取った 12 Jan 2024 受け入れられた 25 Mar 2024 オンラインで公開された 26 Mar 2024
Breast cancer is the most common type of cancer in women worldwide. It can be spread when cancer cells get into the blood or lymph system and then are carried to other parts of the body. As it is one of the leading causes of death among women, this study aims to identify the most relevant risk factors for breast cancer through several prevention methods for early detection. The social impact of breast cancer is so strong that the World Health Organization (WHO) has established 19 October as International Breast Cancer Day. The pink ribbon is the symbol of this important day worldwide. The objective is precisely to raise awareness among the population about the disease and promote access to timely and effective diagnoses, checks, and treatments.
Good prevention should be carried out through behavior or lifestyle modifications (e.g., diet, physical activity, alcohol limitation, etc.). Although, certain risk factors cannot be modified (e.g., aging, family history). Screenings are a fundamental tool to deal with breast cancer, even if sometimes they are not enough as in the case of interval cancers or in cases of particularly ambiguous presentation. Worldwide organizations recommend screening by means of ultrasound, mammography, and magnetic resonance, with appropriate follow-up for an abnormal screening test. To avoid overdiagnosis and overtreatment that can lead to long-term complications and false negatives, these screening differs by recommended ages and frequency. Identification of women at risk for BRCA1 and BRCA2 mutations is also recommended with a referral for genetic testing and, in the presence of dubious lesions, a biopsy is needed. In order to fight this disease, is important to act on time, so, information is fundamental. First of all, the female population should be conscious of risk factors and of the importance of breast examinations from a young age, lastly, they should be aware of the possibility of joining programs of free screening.
Despite the recent advances in diagnosis and treatment for breast cancer, it is still the leading cause of death in women worldwide. In 2020, 2.3 million females were diagnosed with breast cancer worldwide []. The incidence and related mortality from breast cancer continue to grow (about 0.2% cases per year), despite remarkable advances in our understanding of the biology of breast cancer and the availability of better therapeutic options []. The American Cancer Society’s 2022 update estimated that about 287,850 new cases of breast cancer will be diagnosed in US women, with an expected 43,250 deaths presentation [].
It is due to the uncontrolled proliferation of some cells of the mammary gland which, transforming into malignant cells, acquire the ability to migrate and invade surrounding tissues and organs and, over time, even more distant organs. In theory, all cells in the breast can give rise to cancer, but in most cases, cancer originates from glandular cells (from lobules) or from those that form the walls of the ducts.
Breast cancer is a heterogeneous neoplasm determined by a variety of genetic alterations in mammary epithelial cells, leading to huge disease manifestations in individual patients. It can be more or less aggressive; among the most aggressive forms are those with genetic etiopathogenesis, a condition which, in addition to worsening the prognosis, also anticipates the age of presentation [].
Identifying the disease in time is the best way to fight it. Prevention is mainly based on the use of two methods: ultrasound and mammography. However, it must be customized according to the risk, which is calculated by evaluating many factors.
Breast cancer is a multifactorial disease, among the causes some can be some can be preventable as they are correlated with lifestyle, so they can be easily modifiable.
The excess of body fat increases the risk of postmenopausal breast cancer as it becomes the primary site of estrogen production. Fatty tissue also develops chronic low-grade inflammation, increasing the production of free radicals that can damage DNA. This can activate some signaling pathways that induce the expression of some genes that promote the development and progression of cancer. Finally, higher levels of body fat can also cause metabolic changes that make insulin less effective, causing the body to secrete more. Higher levels of insulin increase the signals for tumor growth [-]. Alcohol increases the risk of cancer in proportion to the assumed dose. Among the biological mechanisms activated by excessive alcohol consumption, is the activation of aromatase, an enzyme that stimulates the conversion of androgens into estrogens []. Regarding tobacco consumption, there is consistent evidence of a moderately increased risk of breast cancer in women who smoke []. Ionizing radiation also increases the risk of breast cancer. These can be attributable to occupational exposure or previous radiotherapy to the thoracic region [].
The risk of being affected by breast cancer increases with age. The peak of incidence occurs in women between 50 and 70 years old. It is very rare (7% - 8%) before the age of 40 and exceptionally (< 1%) before 30 years old and hardly ever (< 1%) before 30 years old, even though its incidence is increasing 0.2% per year. [].
In assessing the risk of breast cancer, the evaluation of family history as a threat factor is very important. Almost a quarter of all cases are related to family history. Women, whose mother or sister has or had breast cancer, are more susceptible to this complaint []. Additionally, the risk increases 2.5 times or more in women with two or more first-degree relatives with breast cancer. The inherited susceptibility to breast cancer is relatively attributed to the mutations of breast cancer-related genes such as BRCA1 and BRCA2. A cohort study of over 113,000 women in the UK showed that women with a first-degree relative with breast cancer are 1.75 times more likely to develop the disease than women with no affected relative [].
Also, estrogens play a key role in breast cancer development as early as puberty. All those conditions that increase estrogenic stimulation can lead to an increased risk of developing breast cancer. Many breast cancers are also estrogen-dependent. Risk factors therefore include the use of the contraceptive pill, postmenopausal estrogen replacement therapy, and hormone therapy for ovarian stimulation []. Early menarche and/or late menopause increase the risk of breast cancer because the time during which the breast is subjected to estrogenic stimulation increases. For the same reason, nulliparity increases the risk also because there is no hormonal block for 9 months of pregnancy []. A study published in 2002 [] showed that women with breast cancer had, on average, fewer births than controls (2.2 vs. 2.6). Furthermore, among women with children, there were fewer women who breastfed in the cancer group than in the controls (71% vs. 79%). For those who still breastfed on average of fewer months (9.8 vs. 15.6 months), showed that the relative risk of breast cancer decreased by 4.3% for each 12 months of breastfeeding and by 7.0% for each birth. The breast undergoes transformations during pregnancy and only with breastfeeding does the mammary gland complete its maturation, so the breast cell is more resistant to mutations that can lead to cancer. So last but not least, another risk factor is not breastfeeding. Another risk factor resides in previous breast disease, but also mastitis and benign diseases [] (Graph).
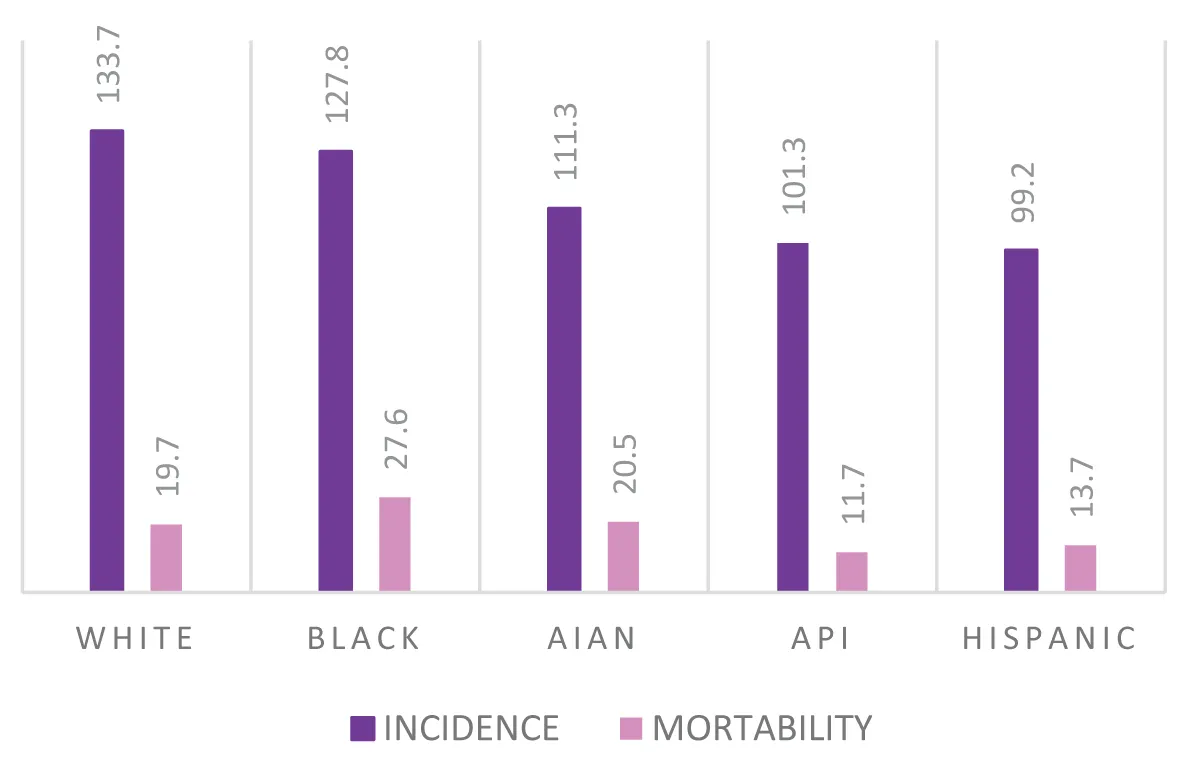
Last but not least, it is worth mentioning the race that affects both the prognosis and the characteristics of the tumor. According to a USA study, in fact, breast cancer incidence rates are highest in white women, followed by black and American Indian/Alaska Native (AIAN) women, and are lowest in Hispanic women and Asian/Pacific Islander women (API).
However, black women have the highest breast cancer death rate, 40% higher than the rate of white women and more than double that of API ones [,].
This is probably due to two different aspects: delay in diagnosis and a higher percentage of more aggressive tumors.
Early diagnosis on average occurs in 57% - 60% of Black, Hispanic, and AIAN women compared to 65% of API women and 68% of white women.
Additionally, black women have the highest rate of high-grade cancers. Black women are twice as likely as other women to be diagnosed with HR-negative/HER2-negative (also called triple-negative) cancers: 19% compared to 11% of Hispanic and AIAN women and 9% of white women and API [].
First, the easiest way to prevent breast cancer is to know the biological mechanism for which cells go through the apoptosis process and especially which are the biomarkers involved. Having a deep knowledge of this, it would be more probable to discover the disease at an early stage so that can be cured.
Stephen Elledge, et al. demonstrated the role of the repressor element 1 (RE-1)-silencing transcription factor (REST) as an oncosoppressor, after a study on RNAi-based screen for tumor-suppressor genes in human mammary epithelial cells. It was found that if the REST’s function in epithelial cells was blocked, a phenotype transformation would occur, such as anchorage-independent growth (Neuman, et al. 2004). At least, Hui Lv, et al. demonstrated the expression of REST in breast cancer tissue by immunohistochemistry assay. Unfortunately, acquiring all the necessary knowledge about the biochemistry of breast cancer takes time, so we really have to build on what we have, starting with the study of the patient in question.
Since breast cancer is a multifactorial disease, it is easy to understand how the risk can therefore be stratified according to the patient; this consequently entails the customization of the preventive procedure.
In fact, preventive examinations will be more in-depth and anticipated for patients with a family history of breast cancer, but above all for those with genetic predisposition for BRCA 1 and BRCA 2 mutations. These are involved in DNA repair mechanisms and are deeply studied as some of their forms mutate and hereditary transmission indicates the risk of developing certain types of cancer, particularly breast and ovarian cancer (William D. Foulkes, et al. 2013).
Preventive health measures must be divided into three levels: primary, secondary, and tertiary prevention [].
The purpose of primary prevention is to prevent the development of pathology takes place.
Secondary prevention should be performed before symptoms appear, so that the disease is recognized and early treated, minimizing adverse consequences. It may include screening programs, such as mammography.
In tertiary prevention, an underlying disease, usually chronic, is treated with the aim of preventing complications or further damage it could cause [].
A good lifestyle is considered the best primary prevention strategy identified thus far. Therefore, the maintenance of healthy body weight, regular physical activity, and moderation of alcohol intake, contribute to the prevention of other tumors and diseases, in addition to breast cancer. A diet that helps maintain the right body weight, rich in fruit, vegetables, cereals, and legumes, containing little red meat and little salt, and almost free of processed meat is recommended []. Further precautions are obviously not to smoke and to exercise regularly []. As far as secondary prevention is concerned, self-examination is certainly one of the practices to be adopted to try to identify a possible tumor as early as possible. This is an examination that women can perform on their own from the age of 20, once a month, one week after the end of their period (since this is the phase in which the breasts are less painful and turgid); if a woman is pregnant or in menopause, the period in which she can self-examine is irrelevant. The American Cancer Society (1990), recommends that all women over the age of 20 have monthly breast self-exams []. The presence of lumps, retraction secretions from the nipple, or other skin alterations must alarm and lead the patient to a breast examination.
The first diagnostic tests to evaluate the health state of the breast are ultrasound (US) and mammography (MMG). The US is a non-invasive method as it uses sound waves at frequencies greater than 20 kHz and does not ionizing radiation which does not harm human health. It is advisable to perform it already starting from the age of 20, once a year. It is a very important examination, especially for dense breasts for which the mammographic examination could not be decisive []. The US is a particularly useful diagnostic modality to distinguish cystic from solid masses and allows the identification of suspicious solid masses that usually require a biopsy. Additionally, ultrasound is the ideal imaging tool to guide biopsy procedures [].
The golden standard for breast cancer diagnosis is definitely MMG, which represents the principal modality of early detection for women at average risk []. Early detection allows the diagnosis of tumors of small sizes with few nodal metastases and less histologic-grade progression, making the treatment more effective []. Literature data show substantial benefits for women who undergo mammography screening versus those not participating in screening [].
The downside of mammography is that it is associated with a small amount of radiation that in the long term can bring adverse effects if is often faced in screening tests. The individual dose may differ depending on breast size and compression. However, with modern mammographic techniques and in particular with full-field digital mammography, the glandular dose has dropped significantly during the last decades and is continuously decreasing [].
Many countries, especially those with higher incomes, have established screening programs based on this method, through which a free checkup is periodically offered to a part of the population that responds to certain characteristics. The frequency and age range of screening varies from country to country and sometimes even from region to region. For that reason, screenings are an excellent aid in reaching a large number of people, but at the same time, the strict rules to be respected make it insufficient. The ACR (American College of Radiology) recommends annual mammography screening starting at the age of 40 for women at average risk, and it is also recommended beyond the age of 70 if a woman’s health permits it [,].
Actually, a cross-sectional study conducted on a total of 415,277 breast cancer deaths in female patients in the United States from 2011 to 2020 found that when screening was recommended at age 50 for the general female population, black women should begin it 8 years earlier, at age 42, while white women could begin at age 51, American Indian or Alaska Native and Hispanic women at age 57 and Asian or Pacific Islander women at age 61 in relation to race-related risk factors [].
Not all countries offer such efficient screenings however, in these cases there will be a greater chance of having the so-called “interval cancers (IC)” which by definition are cancers that become palpable between 2 screening rounds []. The biggest problem is given by the fact that they have rapid growth, so they can be detected by the MMG only at the moment of the diagnosis (“true IC”) []. In some cases, there could be the presence of a carcinogenic mass, but because of a substandard technique or positioning (not including cancer in the image), it cannot be seen, giving rise to a false negative. In addition, it can also happen that the cancer is masked by dense breast tissue, or visible in retrospect but not detected or misinterpreted by the screener. It is therefore suggested to take care of itself individually by carrying out breast examinations annually.
High-risk women should start mammography screening earlier and may benefit from additional screening modalities with contrast-enhanced breast Magnetic Resonance Imaging (MRI) []. However, an add-on strategy (supplementary screening breast US or MRI in addition to mammography (MG) can result in a higher cancer detection rate [-] and an increased false-positive rate [-]. For pregnant women, physical breast examination screenings during pregnancy and breastfeeding are strongly recommended, while mammography (MG), magnetic resonance imaging (MRI), and ultrasound imaging (US) are not considered appropriate for women in this condition [].
Magnetic resonance (MR) imaging is an advanced preventive diagnostic test reserved for healthy women but with an important family history of breast cancer or carriers of a mutation, who therefore have a higher risk of developing breast cancer.
The American Cancer Society guidelines and the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology recommend annual breast MRI screening for women at high risk for breast cancer, which includes BRCA mutation carriers and their untested first-degree relatives; women with Li-Fraumeni syndrome and other high-risk predisposition syndromes; women with a history of thoracic radiation therapy between age 10 and 30 years; and women with 20% or greater lifetime risk of breast cancer based on risk assessment models. Moreover, for women with high risk an annual MRI screening should start at age 25–30 years and annual mammography screening is also recommended, starting preferably at age 30 years old. MRI, on the other hand, poses no risk of radiation-induced cancer and exhibits high sensitivity, and the potential risks from the application of gadolinium-containing contrast media are minimal [].
Genetic implications for breast cancer may be associated with mutations in a specific gene or a series of genes, including the key tumor suppressor gene BRCA (BRCA1 or BRCA2). Such mutations may be inherited (germline) or arise as a result of a combination of genetic and environmental factors (somatic) []. BRCA tests are not expected for all women as a routine screening procedure, but it is strongly suggested for women who have a positive family history of breast cancer, or several relatives suffering from breast and/or ovarian cancer if there is a male member of the family affected by breast cancer (in men it is much rarer and more aggressive) []. However, BRCA mutations are thought to be uncommon in the general population (much less than 1%).
If the presence of a dubious lesion emerges during the breast examination, it is always advisable to analyze its content by biopsy to confirm malignant cases or deny benign ones, and possibly undertake suitable treatment as soon as possible. US, MG, MRI, and positron emission mammography (PEM) are now successfully used to guide the biopsy needle to obtain a proper tissue sample that can be histologically assessed []. Biopsy consists of taking breast tissue material in the form of small cylindrical fragments, taken from the area of the breast in which an anomaly or a suspected neoplastic formation has been identified. The material thus collected is then put to histological examination or other laboratory tests. Complications are possible but rare. However, it is advisable to perform the analysis to confirm the malignant cases or possibly deny the benign ones and undertake a suitable treatment as soon as possible []. In highly risky cases, with confirmed genetic mutation, the most effective measure to reduce breast cancer risk is bilateral mastectomy, although guidelines recommend limiting this to women at substantially increased risk [].
Digital breast tomosynthesis (DBT) is emerging as the standard of care for breast imaging based on a quasi-three-dimensional technique, where multiple low-dose images are obtained over a range of angles, and reconstructed in slices []. The information obtained from the tomosynthesis decreases the clutter effect of overlapping tissue. This allows an improved lesion detection, characterization, and localization. In addition, the quasi-three-dimensional information obtained from the reconstructed DBT data set allows a more efficient imaging work-up than imaging with two-dimensional full-field digital mammography alone. Breast tomosynthesis overcomes some of the limitations of standard mammography, but it is not yet available in all imaging facilities.
Nowadays we hear more and more about artificial intelligence (AI), but in reality, it was mentioned for the first time in 1956 during a conference held at Dartmouth College in Hanover, New Hampshire. Artificial intelligence is the starting point for the development of even more innovative applications such as Machine Learning (ML) and Deep Learning (DL) []. Artificial Intelligence refers to computer systems that simulate or exhibit a specific aspect of human intelligence or intelligent behavior, such as learning, reasoning, and problem-solving [].
The first applications of AI were unsuccessful as scientists tried to model the human mental process based on the prior knowledge of experts, through a series of codified rules.
With the development of ML was introduced a system not explicitly programmed, but is “trained” on the basis of experience [].
Machines capable of learning from experience have thus been developed through specific computational methods that allow them to develop their own internal algorithm, scilicet the relative ability of a machine to learn without being explicitly programmed to do so.
Machine learning is an IT process that involves several phases: through the use of a series of data, called “training dataset”, we proceed with the training of the algorithm which will allow us to obtain an analytical model. Subsequently, using a series of data called “testing dataset”, we proceed with the validation of the model [].
DL, or deep learning, is another approach to machine learning and represents an advanced ML technique. It is a mathematical model that, inspired by the functioning of neurons in the animal brain, uses multilayer artificial neural network models (Artificial Neural Network - ANN) with various processing units and can exploit computational processes.
In summary, the 3 terms should not be confused: AI is the final goal we want to achieve, ML is an approach and DL is an ML technique [].
Inappropriate datasets and poor image quality may limit the conspicuity of the breast lesion’s characters or offer inadequate inputs for the AI system [,].
In the breast sector, the CAD (Computer-aided diagnosis) system has been developed to help radiologists automate the early discovery and diagnosis of breast lesions [].
In 1998, CAD systems utilizing traditional ML were developed and used as a second opinion to analyze patients’ images in mammography and improve radiologists’ performance.
Literature shows that despite the positive impact of CAD systems on breast cancer screening, decreases in specificity and increases in recall rates are also noted [].
The conventional CAD system has been established to aid radiologists, not to be used as a primary screening tool. It is designed and trained to detect specific features that radiologists look for, such as masses or classifications [].
Therefore, radiologists should screen and read the images as carefully as they would without CAD, and then use CAD as a ‘spell checker’ following their own interpretation [].
Several factors may affect the performance of AI-based applications, in particular patient populations such as heterogeneity of the breast cancer risk factors, and imaging characteristics of the populations. The shift from the conventional CAD system to advanced AI tools such as DL-CAD has the potential to reduce false-positive findings, increase diagnostic accuracy, improve radiologist performance, and assist with decision-making
Future randomized controlled trials and cohort studies in large-scale samples with high-quality evidence are required to consider the future use of AI-based applications in breast cancer screening [].
In women who showed a high breast cancer risk due to family history or known genetic mutation, managing risk plays a fundamental role. Chemoprevention represents a prevention method developed for such women. The benefits outweigh the risks of chemoprevention once a woman’s 5-year risk of invasive breast cancer reaches 3% [], moreover, decreases lifetime risk by approximately 50% of the high-risk population []. Chemoprevention involves the daily intake of antiestrogen pills (usually tamoxifen for pre-menopausal women, raloxifene for post-menopausal women) for a 5-year course, as an alternative to surgical prevention options (bilateral prophylactic mastectomy and/or oophorectomy).
To date, the low use of chemoprevention is associated with a variety of reasons. Recent studies evidenced that underlying these gaps there is clinicians’ inability to provide adequate quality medical advice. Both general practitioners and familiar cancer specialists also show no confidence in using risk-prediction models to identify high-risk patients. In this way, prescribing chemoprevention medications implies [-], an underestimate of the benefits and/or overestimation of risks. The perception of (or worry about) low drug efficacy and concerns about side effects are negatively associated with chemoprevention use. Therefore, the use of chemoprevention is associated with anxiety and fear in patients who are not predisposed to treatment.
Breast cancer is the most frequent female neoplasm in all age groups. Despite the continuous increase in the incidence of breast cancer, the mortality rate is lower compared with the past. That was possible thanks to the continuous advances in medicine and screening for early diagnosis.
To date, breast cancer prevention in most parts of the world has largely focused on untargeted population-based educational interventions (such as increasing physical activity and reducing BMI and alcohol intake). These will remain an appropriate component of breast cancer prevention, as these interventions also reduce the risk of other important causes of morbidity.
A key component of optimal precision prevention aims to be a systematic and accurate method of assessing each individual woman to estimate, at the right time and at the right woman, the cancer risk. Screenings are a fundamental tool to deal with breast cancer, even if sometimes they are not enough as in the case of interval cancers or of particularly ambiguous presentation.
Delaying screening until age 45 or 50 can determine an increase in new cases. Screening should continue and without age limits, unless severe comorbidities limit life expectancy.
For prevention programs to be sufficiently robust, given the heterogeneity of risk factors, it is important to enroll diverse patient populations in clinical trials to work toward advancing health equity and to better understand the factors that contribute to racial disparities in mortality. For breast cancer []. We need large studies in well-defined and diverse populations with as many criteria as possible to provide estimates of the burden of mutations in underserved and previously understudied populations [].
The only way to fight this disease is to act in time. For this reason, information is important, warning the women population first of all about the risk factors and about the importance of breast examinations from a young age. Moreover, women should be supported toward decisional aids and referred to specialists who can discuss individual risk and risk-management options and, finally, the possibility of joining the programs of free screenings.
The world of medicine has now understood the importance of the impact of breast cancer on modern society and this is why multidisciplinary teams have been raised in many hospitals. They are made up of radiologists, oncologists, general and plastic surgeons, and other specialist figures who together form the so-called Breast Units. These units are entirely dedicated to the prevention and treatment of breast cancer. Better public health education and primary preventive strategies should be used as the main public health intervention.
Arnold M, Morgan E, Rumgay H, Mafra A, Singh D, Laversanne M, Vignat J, Gralow JR, Cardoso F, Siesling S, Soerjomataram I. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast. 2022 Dec;66:15-23. doi: 10.1016/j.breast.2022.08.010. Epub 2022 Sep 2. PMID: 36084384; PMCID: PMC9465273.
Mayrovitz HN, editor. Breast Cancer [Internet]. Brisbane (AU): Exon Publications; 2022 Aug 6. PMID: 36121977.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022 Jan;72(1):7-33. doi: 10.3322/caac.21708. Epub 2022 Jan 12. PMID: 35020204.
Tao Z, Shi A, Lu C, Song T, Zhang Z, Zhao J. Breast Cancer: Epidemiology and Etiology. Cell Biochem Biophys. 2015 Jun;72(2):333-8. doi: 10.1007/s12013-014-0459-6. PMID: 25543329.
Lumachi F, Ermani M, Basso S. (2023). ISBN: 8879998609.
Sun YS, Zhao Z, Yang ZN, Xu F, Lu HJ, Zhu ZY, Shi W, Jiang J, Yao PP, Zhu HP. Risk Factors and Preventions of Breast Cancer. Int J Biol Sci. 2017 Nov 1;13(11):1387-1397. doi: 10.7150/ijbs.21635. PMID: 29209143; PMCID: PMC5715522.
Gompel A. Hormones et cancers du sein [Hormone and breast cancer]. Presse Med. 2019 Oct;48(10):1085-1091. French. doi: 10.1016/j.lpm.2019.09.021. Epub 2019 Oct 26. PMID: 31662219.
Britt KL, Cuzick J, Phillips KA. Key steps for effective breast cancer prevention. Nat Rev Cancer. 2020 Aug;20(8):417-436. doi: 10.1038/s41568-020-0266-x. Epub 2020 Jun 11. PMID: 32528185.
Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50302 women with breast cancer and 96973 women without the disease. Lancet. 2002 Jul 20;360(9328):187-95. doi: 10.1016/S0140-6736(02)09454-0. PMID: 12133652.
Wiseman M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc. 2008 Aug;67(3):253-6. doi: 10.1017/S002966510800712X. Epub 2008 May 1. PMID: 18452640.
Frydenberg H, Flote VG, Larsson IM, Barrett ES, Furberg AS, Ursin G, Wilsgaard T, Ellison PT, McTiernan A, Hjartåker A, Jasienska G, Thune I. Alcohol consumption, endogenous estrogen and mammographic density among premenopausal women. Breast Cancer Res. 2015 Aug 7;17(1):103. doi: 10.1186/s13058-015-0620-1. PMID: 26246001; PMCID: PMC4531831.
Macacu A, Autier P, Boniol M, Boyle P. Active and passive smoking and risk of breast cancer: a meta-analysis. Breast Cancer Res Treat. 2015 Nov;154(2):213-24. doi: 10.1007/s10549-015-3628-4. Epub 2015 Nov 6. PMID: 26546245.
Ng AK, Travis LB. Radiation therapy and breast cancer risk. J Natl Compr Canc Netw. 2009 Nov;7(10):1121-8. doi: 10.6004/jnccn.2009.0073. PMID: 19930978.
Eidemüller M, Holmberg E, Lundell M, Karlsson P. Evidence for Increased Susceptibility to Breast Cancer From Exposure to Ionizing Radiation Due to a Familial History of Breast Cancer: Results From the Swedish Hemangioma Cohort. Am J Epidemiol. 2021 Jan 4;190(1):76-84. doi: 10.1093/aje/kwaa163. PMID: 32735015; PMCID: PMC7784527.
Taparra K, Miller RC, Deville C Jr. Navigating Native Hawaiian and Pacific Islander Cancer Disparities From a Cultural and Historical Perspective. JCO Oncol Pract. 2021 Mar;17(3):130-134. doi: 10.1200/OP.20.00831. Epub 2021 Jan 26. PMID: 33497251.
Medina HN, Callahan KE, Morris CR, Thompson CA, Siweya A, Pinheiro PS. Cancer Mortality Disparities among Asian American and Native Hawaiian/Pacific Islander Populations in California. Cancer Epidemiol Biomarkers Prev. 2021 Jul;30(7):1387-1396. doi: 10.1158/1055-9965.EPI-20-1528. Epub 2021 Apr 20. PMID: 33879454; PMCID: PMC8254771.
Giaquinto AN, Sung H, Miller KD, Kramer JL, Newman LA, Minihan A, Jemal A, Siegel RL. Breast Cancer Statistics, 2022. CA Cancer J Clin. 2022 Nov;72(6):524-541. doi: 10.3322/caac.21754. Epub 2022 Oct 3. PMID: 36190501.
Al-Amri AM. Prevention of breast cancer. J Family Community Med. 2005 May;12(2):71-4. PMID: 23012078; PMCID: PMC3410124.
American Cancer Society (1990). Cancer Facts and Figures. Atlanta American Cancer Society, USA, 56.
Lundberg P, Forsgren MF, Tellman J, Kihlberg J, Rzepecka A, Dabrosin C. Breast density is strongly associated with multiparametric magnetic resonance imaging biomarkers and pro-tumorigenic proteins in situ. Br J Cancer. 2022 Nov;127(11):2025-2033. doi: 10.1038/s41416-022-01976-3. Epub 2022 Sep 22. PMID: 36138072; PMCID: PMC9681775.
Sood R, Rositch AF, Shakoor D, Ambinder E, Pool KL, Pollack E, Mollura DJ, Mullen LA, Harvey SC. Ultrasound for Breast Cancer Detection Globally: A Systematic Review and Meta-Analysis. J Glob Oncol. 2019 Aug;5:1-17. doi: 10.1200/JGO.19.00127. PMID: 31454282; PMCID: PMC6733207.
Heywang-Köbrunner SH, Hacker A, Sedlacek S. Advantages and Disadvantages of Mammography Screening. Breast Care (Basel). 2011;6(3):199-207. doi: 10.1159/000329005. Epub 2011 May 27. PMID: 21779225; PMCID: PMC3132967.
Duffy SW, Tabár L, Yen AM, Dean PB, Smith RA, Jonsson H, Törnberg S, Chen SL, Chiu SY, Fann JC, Ku MM, Wu WY, Hsu CY, Chen YC, Svane G, Azavedo E, Grundström H, Sundén P, Leifland K, Frodis E, Ramos J, Epstein B, Åkerlund A, Sundbom A, Bordás P, Wallin H, Starck L, Björkgren A, Carlson S, Fredriksson I, Ahlgren J, Öhman D, Holmberg L, Chen TH. Mammography screening reduces rates of advanced and fatal breast cancers: Results in 549,091 women. Cancer. 2020 Jul 1;126(13):2971-2979. doi: 10.1002/cncr.32859. Epub 2020 May 11. PMID: 32390151; PMCID: PMC7318598.
Suleiman ME, Brennan PC, McEntee MF. Mean glandular dose in digital mammography: a dose calculation method comparison. J Med Imaging (Bellingham). 2017 Jan;4(1):013502. doi: 10.1117/1.JMI.4.1.013502. Epub 2017 Jan 24. PMID: 28149921; PMCID: PMC5260632.
ACR Appropriateness Criteria. https://www.acr. org/Clinical-Resources/ACR-Appropriateness-Criteria. Accessed February 28, 2021.
Chen T, Kharazmi E, Fallah M. Race and Ethnicity-Adjusted Age Recommendation for Initiating Breast Cancer Screening. JAMA Netw Open. 2023 Apr 3;6(4):e238893. doi: 10.1001/jamanetworkopen.2023.8893. PMID: 37074714; PMCID: PMC10116360.
Monticciolo DL, Newell MS, Moy L, Niell B, Monsees B, Sickles EA. Breast Cancer Screening in Women at Higher-Than-Average Risk: Recommendations From the ACR. J Am Coll Radiol. 2018 Mar;15(3 Pt A):408-414. doi: 10.1016/j.jacr.2017.11.034. Epub 2018 Jan 19. PMID: 29371086.
Gordon PB. The Impact of Dense Breasts on the Stage of Breast Cancer at Diagnosis: A Review and Options for Supplemental Screening. Curr Oncol. 2022 May 17;29(5):3595-3636. doi: 10.3390/curroncol29050291. PMID: 35621681; PMCID: PMC9140155.
Berg WA, Zhang Z, Lehrer D, Jong RA, Pisano ED, Barr RG, Böhm-Vélez M, Mahoney MC, Evans WP 3rd, Larsen LH, Morton MJ, Mendelson EB, Farria DM, Cormack JB, Marques HS, Adams A, Yeh NM, Gabrielli G; ACRIN 6666 Investigators. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012 Apr 4;307(13):1394-404. doi: 10.1001/jama.2012.388. PMID: 22474203; PMCID: PMC3891886.
Riedl CC, Luft N, Bernhart C, Weber M, Bernathova M, Tea MK, Rudas M, Singer CF, Helbich TH. Triple-modality screening trial for familial breast cancer underlines the importance of magnetic resonance imaging and questions the role of mammography and ultrasound regardless of patient mutation status, age, and breast density. J Clin Oncol. 2015 Apr 1;33(10):1128-35. doi: 10.1200/JCO.2014.56.8626. Epub 2015 Feb 23. PMID: 25713430; PMCID: PMC5526626.
Vreemann S, van Zelst JCM, Schlooz-Vries M, Bult P, Hoogerbrugge N, Karssemeijer N, Gubern-Mérida A, Mann RM. The added value of mammography in different age-groups of women with and without BRCA mutation screened with breast MRI. Breast Cancer Res. 2018 Aug 3;20(1):84. doi: 10.1186/s13058-018-1019-6. PMID: 30075794; PMCID: PMC6091096.
Sung JS, Stamler S, Brooks J, Kaplan J, Huang T, Dershaw DD, Lee CH, Morris EA, Comstock CE. Breast Cancers Detected at Screening MR Imaging and Mammography in Patients at High Risk: Method of Detection Reflects Tumor Histopathologic Results. Radiology. 2016 Sep;280(3):716-22. doi: 10.1148/radiol.2016151419. Epub 2016 Apr 20. PMID: 27097237; PMCID: PMC5006733.
Lee JM, Arao RF, Sprague BL, Kerlikowske K, Lehman CD, Smith RA, Henderson LM, Rauscher GH, Miglioretti DL. Performance of Screening Ultrasonography as an Adjunct to Screening Mammography in Women Across the Spectrum of Breast Cancer Risk. JAMA Intern Med. 2019 May 1;179(5):658-667. doi: 10.1001/jamainternmed.2018.8372. Erratum in: JAMA Intern Med. 2019 Apr 29;: PMID: 30882843; PMCID: PMC6503561.
Ożegalska-Trybalska J. Plagiarism and self-plagiarism – facts and myths. Nowotwory. Journal of Oncology. 2021; 71(1): 71–72. doi: 10.5603/ NJO.2021.0012.
Saccarelli CR, Bitencourt AGV, Morris EA. Breast Cancer Screening in High-Risk Women: Is MRI Alone Enough? J Natl Cancer Inst. 2020 Feb 1;112(2):121-122. doi: 10.1093/jnci/djz130. PMID: 31233125; PMCID: PMC7019094.
Engel C, Fischer C. Breast cancer risks and risk prediction models. Breast Care (Basel). 2015 Feb;10(1):7-12. doi: 10.1159/000376600. PMID: 25960719; PMCID: PMC4395818.
Taylor MR. Genetic testing for inherited breast and ovarian cancer syndromes: important concepts for the primary care physician. Postgrad Med J. 2001 Jan;77(903):11-5. doi: 10.1136/pmj.77.903.11. PMID: 11123386; PMCID: PMC1741878.
Guo R, Lu G, Qin B, Fei B. Ultrasound Imaging Technologies for Breast Cancer Detection and Management: A Review. Ultrasound Med Biol. 2018 Jan;44(1):37-70. doi: 10.1016/j.ultrasmedbio.2017.09.012. Epub 2017 Oct 26. PMID: 29107353; PMCID: PMC6169997.
Rebbeck TR, Friebel T, Lynch HT, Neuhausen SL, van 't Veer L, Garber JE, Evans GR, Narod SA, Isaacs C, Matloff E, Daly MB, Olopade OI, Weber BL. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol. 2004 Mar 15;22(6):1055-62. doi: 10.1200/JCO.2004.04.188. Epub 2004 Feb 23. PMID: 14981104.
Andresen SL. John McCarthy: father of AI. IEEE Intelligent Systems 2002; 17:84-5.
McCarthy J. The Dartmouth summer research project on artificial intelligence. Artificial intelligence: past, present, and future. 1956.
Choi RY, Coyner AS, Kalpathy-Cramer J, Chiang MF, Campbell JP. Introduction to Machine Learning, Neural Networks, and Deep Learning. Transl Vis Sci Technol. 2020 Feb 27;9(2):14. doi: 10.1167/tvst.9.2.14. PMID: 32704420; PMCID: PMC7347027.
Guerranti R, Padoan A, Angeletti D, Foracchia M. Introduzione ai big data e all’Intelligenza artificiale in medicina di laboratorio. Biochim Clin. 2021; 45: 57-67.
LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015 May 28;521(7553):436-44. doi: 10.1038/nature14539. PMID: 26017442.
Reiner B. Contextualizing Causation of Uncertainty in Medical Reporting. J Am Coll Radiol. 2018 Feb;15(2):325-327. doi: 10.1016/j.jacr.2017.08.019. Epub 2017 Sep 22. PMID: 28958724.
Sabottke CF, Spieler BM. The Effect of Image Resolution on Deep Learning in Radiography. Radiol Artif Intell. 2020 Jan 22;2(1):e190015. doi: 10.1148/ryai.2019190015. PMID: 33937810; PMCID: PMC8017385.
Jalalian A, Mashohor S, Mahmud R, Karasfi B, Saripan MIB, Ramli ARB. Foundation and methodologies in computer-aided diagnosis systems for breast cancer detection. EXCLI J. 2017 Feb 20;16:113-137. doi: 10.17179/excli2016-701. PMID: 28435432; PMCID: PMC5379115.
Ou WC, Polat D, Dogan BE. Deep learning in breast radiology: current progress and future directions. Eur Radiol. 2021 Jul;31(7):4872-4885. doi: 10.1007/s00330-020-07640-9. Epub 2021 Jan 15. PMID: 33449174.
Kohli A, Jha S. Why CAD Failed in Mammography. J Am Coll Radiol. 2018 Mar;15(3 Pt B):535-537. doi: 10.1016/j.jacr.2017.12.029. Epub 2018 Feb 3. PMID: 29398499.
Chan HP, Hadjiiski LM, Samala RK. Computer-aided diagnosis in the era of deep learning. Med Phys. 2020 Jun;47(5):e218-e227. doi: 10.1002/mp.13764. PMID: 32418340; PMCID: PMC7293164.
Alsharif WM. The utilization of artificial intelligence applications to improve breast cancer detection and prognosis. Saudi Med J. 2023 Feb;44(2):119-127. doi: 10.15537/smj.2023.44.2.20220611. PMID: 36773967; PMCID: PMC9987701.
Farkas A, Vanderberg R, Merriam S, DiNardo D. Breast Cancer Chemoprevention: A Practical Guide for the Primary Care Provider. J Womens Health (Larchmt). 2020 Jan;29(1):46-56. doi: 10.1089/jwh.2018.7643. Epub 2019 Sep 27. PMID: 31560601.
National Cancer Institute. National Cancer Institute Fact Sheet: Breast cancer risk in American women. http://www.cancer.gov/cancertopics/ factsheet/detection/probability-breast-cancer.
Smith SG, Foy R, McGowan JA, Kobayashi LC, de Censi A, DeCensi A, Brown K, Side L, Cuzick J. Prescribing tamoxifen in primary care for the prevention of breast cancer: a national online survey of GPs' attitudes. Br J Gen Pract. 2017 Jun;67(659):e414-e427. doi: 10.3399/bjgp17X689377. Epub 2017 Feb 13. Erratum in: Br J Gen Pract. 2018 Oct;68(675):468. DeCensi A [corrected to de Censi A]. PMID: 28193617; PMCID: PMC5442957.
Bidassie B, Kovach A, Vallette MA, Merriman J, Park YA, Aggarwal A, Colonna S. Breast Cancer Risk Assessment and Chemoprevention Use Among Veterans Affairs Primary Care Providers: A National Online Survey. Mil Med. 2020 Mar 2;185(3-4):512-518. doi: 10.1093/milmed/usz291. PMID: 31865375.
Lee SI, Curtis H, Qureshi S, et al. Specialist recommendation for chemoprevention medications in patients at familial risk of breast cancer: a cross-sectional survey in England. J Community Genet. 2021; 12(1):111–20.
Kyalwazi B, Yau C, Campbell MJ, Yoshimatsu TF, Chien AJ, Wallace AM, Forero-Torres A, Pusztai L, Ellis ED, Albain KS, Blaes AH, Haley BB, Boughey JC, Elias AD, Clark AS, Isaacs CJ, Nanda R, Han HS, Yung RL, Tripathy D, Edmiston KK, Viscusi RK, Northfelt DW, Khan QJ, Asare SM, Wilson A, Hirst GL, Lu R, Symmans WF, Yee D, DeMichele AM, van 't Veer LJ, Esserman LJ, Olopade OI. Race, Gene Expression Signatures, and Clinical Outcomes of Patients With High-Risk Early Breast Cancer. JAMA Netw Open. 2023 Dec 1;6(12):e2349646. doi: 10.1001/jamanetworkopen.2023.49646. PMID: 38153734; PMCID: PMC10755617.
Omilian AR, Wei L, Hong CC, Bandera EV, Liu S, Khoury T, Ambrosone CB, Yao S. Somatic mutations of triple-negative breast cancer: a comparison between Black and White women. Breast Cancer Res Treat. 2020 Jul;182(2):503-509. doi: 10.1007/s10549-020-05693-4. Epub 2020 May 21. PMID: 32441016; PMCID: PMC7313393.
Grattagliano Z, Grattagliano A. Breast Cancer: The Road to a Personalized Prevention. IgMin Res. 26 Mar, 2024; 2(3): 163-170. IgMin ID: igmin160; DOI:10.61927/igmin160; Available at: igmin.link/p160
次のリンクを共有した人は、このコンテンツを読むことができます:
1Interdisciplinary Department of Medicine, Section of Diagnostic Imaging, University of Bari Medical School “Aldo Moro”, Piazza Giulio Cesare 11, Bari, 70124, Italy
2University of Rome Tor Vergata, Department of Chemical Sciences and Technologies, Via della Ricerca Scientifi ca 1, 00133, Rome, Italy
Address Correspondence:
Asia Grattagliano, University of Rome Tor Vergata, Department of Chemical Sciences and Technologies, Via della Ricerca Scientifica 1, 00133, Rome, Italy, Email: [email protected]
How to cite this article:
Grattagliano Z, Grattagliano A. Breast Cancer: The Road to a Personalized Prevention. IgMin Res. 26 Mar, 2024; 2(3): 163-170. IgMin ID: igmin160; DOI:10.61927/igmin160; Available at: igmin.link/p160
Copyright: © 2024 Grattagliano Z, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
 : ...
: ...
 : Graphical abstract...
: Graphical abstract...
Arnold M, Morgan E, Rumgay H, Mafra A, Singh D, Laversanne M, Vignat J, Gralow JR, Cardoso F, Siesling S, Soerjomataram I. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast. 2022 Dec;66:15-23. doi: 10.1016/j.breast.2022.08.010. Epub 2022 Sep 2. PMID: 36084384; PMCID: PMC9465273.
Mayrovitz HN, editor. Breast Cancer [Internet]. Brisbane (AU): Exon Publications; 2022 Aug 6. PMID: 36121977.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022 Jan;72(1):7-33. doi: 10.3322/caac.21708. Epub 2022 Jan 12. PMID: 35020204.
Tao Z, Shi A, Lu C, Song T, Zhang Z, Zhao J. Breast Cancer: Epidemiology and Etiology. Cell Biochem Biophys. 2015 Jun;72(2):333-8. doi: 10.1007/s12013-014-0459-6. PMID: 25543329.
Lumachi F, Ermani M, Basso S. (2023). ISBN: 8879998609.
Sun YS, Zhao Z, Yang ZN, Xu F, Lu HJ, Zhu ZY, Shi W, Jiang J, Yao PP, Zhu HP. Risk Factors and Preventions of Breast Cancer. Int J Biol Sci. 2017 Nov 1;13(11):1387-1397. doi: 10.7150/ijbs.21635. PMID: 29209143; PMCID: PMC5715522.
Gompel A. Hormones et cancers du sein [Hormone and breast cancer]. Presse Med. 2019 Oct;48(10):1085-1091. French. doi: 10.1016/j.lpm.2019.09.021. Epub 2019 Oct 26. PMID: 31662219.
Britt KL, Cuzick J, Phillips KA. Key steps for effective breast cancer prevention. Nat Rev Cancer. 2020 Aug;20(8):417-436. doi: 10.1038/s41568-020-0266-x. Epub 2020 Jun 11. PMID: 32528185.
Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50302 women with breast cancer and 96973 women without the disease. Lancet. 2002 Jul 20;360(9328):187-95. doi: 10.1016/S0140-6736(02)09454-0. PMID: 12133652.
Wiseman M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc. 2008 Aug;67(3):253-6. doi: 10.1017/S002966510800712X. Epub 2008 May 1. PMID: 18452640.
Frydenberg H, Flote VG, Larsson IM, Barrett ES, Furberg AS, Ursin G, Wilsgaard T, Ellison PT, McTiernan A, Hjartåker A, Jasienska G, Thune I. Alcohol consumption, endogenous estrogen and mammographic density among premenopausal women. Breast Cancer Res. 2015 Aug 7;17(1):103. doi: 10.1186/s13058-015-0620-1. PMID: 26246001; PMCID: PMC4531831.
Macacu A, Autier P, Boniol M, Boyle P. Active and passive smoking and risk of breast cancer: a meta-analysis. Breast Cancer Res Treat. 2015 Nov;154(2):213-24. doi: 10.1007/s10549-015-3628-4. Epub 2015 Nov 6. PMID: 26546245.
Ng AK, Travis LB. Radiation therapy and breast cancer risk. J Natl Compr Canc Netw. 2009 Nov;7(10):1121-8. doi: 10.6004/jnccn.2009.0073. PMID: 19930978.
Eidemüller M, Holmberg E, Lundell M, Karlsson P. Evidence for Increased Susceptibility to Breast Cancer From Exposure to Ionizing Radiation Due to a Familial History of Breast Cancer: Results From the Swedish Hemangioma Cohort. Am J Epidemiol. 2021 Jan 4;190(1):76-84. doi: 10.1093/aje/kwaa163. PMID: 32735015; PMCID: PMC7784527.
Taparra K, Miller RC, Deville C Jr. Navigating Native Hawaiian and Pacific Islander Cancer Disparities From a Cultural and Historical Perspective. JCO Oncol Pract. 2021 Mar;17(3):130-134. doi: 10.1200/OP.20.00831. Epub 2021 Jan 26. PMID: 33497251.
Medina HN, Callahan KE, Morris CR, Thompson CA, Siweya A, Pinheiro PS. Cancer Mortality Disparities among Asian American and Native Hawaiian/Pacific Islander Populations in California. Cancer Epidemiol Biomarkers Prev. 2021 Jul;30(7):1387-1396. doi: 10.1158/1055-9965.EPI-20-1528. Epub 2021 Apr 20. PMID: 33879454; PMCID: PMC8254771.
Giaquinto AN, Sung H, Miller KD, Kramer JL, Newman LA, Minihan A, Jemal A, Siegel RL. Breast Cancer Statistics, 2022. CA Cancer J Clin. 2022 Nov;72(6):524-541. doi: 10.3322/caac.21754. Epub 2022 Oct 3. PMID: 36190501.
Al-Amri AM. Prevention of breast cancer. J Family Community Med. 2005 May;12(2):71-4. PMID: 23012078; PMCID: PMC3410124.
American Cancer Society (1990). Cancer Facts and Figures. Atlanta American Cancer Society, USA, 56.
Lundberg P, Forsgren MF, Tellman J, Kihlberg J, Rzepecka A, Dabrosin C. Breast density is strongly associated with multiparametric magnetic resonance imaging biomarkers and pro-tumorigenic proteins in situ. Br J Cancer. 2022 Nov;127(11):2025-2033. doi: 10.1038/s41416-022-01976-3. Epub 2022 Sep 22. PMID: 36138072; PMCID: PMC9681775.
Sood R, Rositch AF, Shakoor D, Ambinder E, Pool KL, Pollack E, Mollura DJ, Mullen LA, Harvey SC. Ultrasound for Breast Cancer Detection Globally: A Systematic Review and Meta-Analysis. J Glob Oncol. 2019 Aug;5:1-17. doi: 10.1200/JGO.19.00127. PMID: 31454282; PMCID: PMC6733207.
Heywang-Köbrunner SH, Hacker A, Sedlacek S. Advantages and Disadvantages of Mammography Screening. Breast Care (Basel). 2011;6(3):199-207. doi: 10.1159/000329005. Epub 2011 May 27. PMID: 21779225; PMCID: PMC3132967.
Duffy SW, Tabár L, Yen AM, Dean PB, Smith RA, Jonsson H, Törnberg S, Chen SL, Chiu SY, Fann JC, Ku MM, Wu WY, Hsu CY, Chen YC, Svane G, Azavedo E, Grundström H, Sundén P, Leifland K, Frodis E, Ramos J, Epstein B, Åkerlund A, Sundbom A, Bordás P, Wallin H, Starck L, Björkgren A, Carlson S, Fredriksson I, Ahlgren J, Öhman D, Holmberg L, Chen TH. Mammography screening reduces rates of advanced and fatal breast cancers: Results in 549,091 women. Cancer. 2020 Jul 1;126(13):2971-2979. doi: 10.1002/cncr.32859. Epub 2020 May 11. PMID: 32390151; PMCID: PMC7318598.
Suleiman ME, Brennan PC, McEntee MF. Mean glandular dose in digital mammography: a dose calculation method comparison. J Med Imaging (Bellingham). 2017 Jan;4(1):013502. doi: 10.1117/1.JMI.4.1.013502. Epub 2017 Jan 24. PMID: 28149921; PMCID: PMC5260632.
ACR Appropriateness Criteria. https://www.acr. org/Clinical-Resources/ACR-Appropriateness-Criteria. Accessed February 28, 2021.
Chen T, Kharazmi E, Fallah M. Race and Ethnicity-Adjusted Age Recommendation for Initiating Breast Cancer Screening. JAMA Netw Open. 2023 Apr 3;6(4):e238893. doi: 10.1001/jamanetworkopen.2023.8893. PMID: 37074714; PMCID: PMC10116360.
Monticciolo DL, Newell MS, Moy L, Niell B, Monsees B, Sickles EA. Breast Cancer Screening in Women at Higher-Than-Average Risk: Recommendations From the ACR. J Am Coll Radiol. 2018 Mar;15(3 Pt A):408-414. doi: 10.1016/j.jacr.2017.11.034. Epub 2018 Jan 19. PMID: 29371086.
Gordon PB. The Impact of Dense Breasts on the Stage of Breast Cancer at Diagnosis: A Review and Options for Supplemental Screening. Curr Oncol. 2022 May 17;29(5):3595-3636. doi: 10.3390/curroncol29050291. PMID: 35621681; PMCID: PMC9140155.
Berg WA, Zhang Z, Lehrer D, Jong RA, Pisano ED, Barr RG, Böhm-Vélez M, Mahoney MC, Evans WP 3rd, Larsen LH, Morton MJ, Mendelson EB, Farria DM, Cormack JB, Marques HS, Adams A, Yeh NM, Gabrielli G; ACRIN 6666 Investigators. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012 Apr 4;307(13):1394-404. doi: 10.1001/jama.2012.388. PMID: 22474203; PMCID: PMC3891886.
Riedl CC, Luft N, Bernhart C, Weber M, Bernathova M, Tea MK, Rudas M, Singer CF, Helbich TH. Triple-modality screening trial for familial breast cancer underlines the importance of magnetic resonance imaging and questions the role of mammography and ultrasound regardless of patient mutation status, age, and breast density. J Clin Oncol. 2015 Apr 1;33(10):1128-35. doi: 10.1200/JCO.2014.56.8626. Epub 2015 Feb 23. PMID: 25713430; PMCID: PMC5526626.
Vreemann S, van Zelst JCM, Schlooz-Vries M, Bult P, Hoogerbrugge N, Karssemeijer N, Gubern-Mérida A, Mann RM. The added value of mammography in different age-groups of women with and without BRCA mutation screened with breast MRI. Breast Cancer Res. 2018 Aug 3;20(1):84. doi: 10.1186/s13058-018-1019-6. PMID: 30075794; PMCID: PMC6091096.
Sung JS, Stamler S, Brooks J, Kaplan J, Huang T, Dershaw DD, Lee CH, Morris EA, Comstock CE. Breast Cancers Detected at Screening MR Imaging and Mammography in Patients at High Risk: Method of Detection Reflects Tumor Histopathologic Results. Radiology. 2016 Sep;280(3):716-22. doi: 10.1148/radiol.2016151419. Epub 2016 Apr 20. PMID: 27097237; PMCID: PMC5006733.
Lee JM, Arao RF, Sprague BL, Kerlikowske K, Lehman CD, Smith RA, Henderson LM, Rauscher GH, Miglioretti DL. Performance of Screening Ultrasonography as an Adjunct to Screening Mammography in Women Across the Spectrum of Breast Cancer Risk. JAMA Intern Med. 2019 May 1;179(5):658-667. doi: 10.1001/jamainternmed.2018.8372. Erratum in: JAMA Intern Med. 2019 Apr 29;: PMID: 30882843; PMCID: PMC6503561.
Ożegalska-Trybalska J. Plagiarism and self-plagiarism – facts and myths. Nowotwory. Journal of Oncology. 2021; 71(1): 71–72. doi: 10.5603/ NJO.2021.0012.
Saccarelli CR, Bitencourt AGV, Morris EA. Breast Cancer Screening in High-Risk Women: Is MRI Alone Enough? J Natl Cancer Inst. 2020 Feb 1;112(2):121-122. doi: 10.1093/jnci/djz130. PMID: 31233125; PMCID: PMC7019094.
Engel C, Fischer C. Breast cancer risks and risk prediction models. Breast Care (Basel). 2015 Feb;10(1):7-12. doi: 10.1159/000376600. PMID: 25960719; PMCID: PMC4395818.
Taylor MR. Genetic testing for inherited breast and ovarian cancer syndromes: important concepts for the primary care physician. Postgrad Med J. 2001 Jan;77(903):11-5. doi: 10.1136/pmj.77.903.11. PMID: 11123386; PMCID: PMC1741878.
Guo R, Lu G, Qin B, Fei B. Ultrasound Imaging Technologies for Breast Cancer Detection and Management: A Review. Ultrasound Med Biol. 2018 Jan;44(1):37-70. doi: 10.1016/j.ultrasmedbio.2017.09.012. Epub 2017 Oct 26. PMID: 29107353; PMCID: PMC6169997.
Rebbeck TR, Friebel T, Lynch HT, Neuhausen SL, van 't Veer L, Garber JE, Evans GR, Narod SA, Isaacs C, Matloff E, Daly MB, Olopade OI, Weber BL. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol. 2004 Mar 15;22(6):1055-62. doi: 10.1200/JCO.2004.04.188. Epub 2004 Feb 23. PMID: 14981104.
Andresen SL. John McCarthy: father of AI. IEEE Intelligent Systems 2002; 17:84-5.
McCarthy J. The Dartmouth summer research project on artificial intelligence. Artificial intelligence: past, present, and future. 1956.
Choi RY, Coyner AS, Kalpathy-Cramer J, Chiang MF, Campbell JP. Introduction to Machine Learning, Neural Networks, and Deep Learning. Transl Vis Sci Technol. 2020 Feb 27;9(2):14. doi: 10.1167/tvst.9.2.14. PMID: 32704420; PMCID: PMC7347027.
Guerranti R, Padoan A, Angeletti D, Foracchia M. Introduzione ai big data e all’Intelligenza artificiale in medicina di laboratorio. Biochim Clin. 2021; 45: 57-67.
LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015 May 28;521(7553):436-44. doi: 10.1038/nature14539. PMID: 26017442.
Reiner B. Contextualizing Causation of Uncertainty in Medical Reporting. J Am Coll Radiol. 2018 Feb;15(2):325-327. doi: 10.1016/j.jacr.2017.08.019. Epub 2017 Sep 22. PMID: 28958724.
Sabottke CF, Spieler BM. The Effect of Image Resolution on Deep Learning in Radiography. Radiol Artif Intell. 2020 Jan 22;2(1):e190015. doi: 10.1148/ryai.2019190015. PMID: 33937810; PMCID: PMC8017385.
Jalalian A, Mashohor S, Mahmud R, Karasfi B, Saripan MIB, Ramli ARB. Foundation and methodologies in computer-aided diagnosis systems for breast cancer detection. EXCLI J. 2017 Feb 20;16:113-137. doi: 10.17179/excli2016-701. PMID: 28435432; PMCID: PMC5379115.
Ou WC, Polat D, Dogan BE. Deep learning in breast radiology: current progress and future directions. Eur Radiol. 2021 Jul;31(7):4872-4885. doi: 10.1007/s00330-020-07640-9. Epub 2021 Jan 15. PMID: 33449174.
Kohli A, Jha S. Why CAD Failed in Mammography. J Am Coll Radiol. 2018 Mar;15(3 Pt B):535-537. doi: 10.1016/j.jacr.2017.12.029. Epub 2018 Feb 3. PMID: 29398499.
Chan HP, Hadjiiski LM, Samala RK. Computer-aided diagnosis in the era of deep learning. Med Phys. 2020 Jun;47(5):e218-e227. doi: 10.1002/mp.13764. PMID: 32418340; PMCID: PMC7293164.
Alsharif WM. The utilization of artificial intelligence applications to improve breast cancer detection and prognosis. Saudi Med J. 2023 Feb;44(2):119-127. doi: 10.15537/smj.2023.44.2.20220611. PMID: 36773967; PMCID: PMC9987701.
Farkas A, Vanderberg R, Merriam S, DiNardo D. Breast Cancer Chemoprevention: A Practical Guide for the Primary Care Provider. J Womens Health (Larchmt). 2020 Jan;29(1):46-56. doi: 10.1089/jwh.2018.7643. Epub 2019 Sep 27. PMID: 31560601.
National Cancer Institute. National Cancer Institute Fact Sheet: Breast cancer risk in American women. http://www.cancer.gov/cancertopics/ factsheet/detection/probability-breast-cancer.
Smith SG, Foy R, McGowan JA, Kobayashi LC, de Censi A, DeCensi A, Brown K, Side L, Cuzick J. Prescribing tamoxifen in primary care for the prevention of breast cancer: a national online survey of GPs' attitudes. Br J Gen Pract. 2017 Jun;67(659):e414-e427. doi: 10.3399/bjgp17X689377. Epub 2017 Feb 13. Erratum in: Br J Gen Pract. 2018 Oct;68(675):468. DeCensi A [corrected to de Censi A]. PMID: 28193617; PMCID: PMC5442957.
Bidassie B, Kovach A, Vallette MA, Merriman J, Park YA, Aggarwal A, Colonna S. Breast Cancer Risk Assessment and Chemoprevention Use Among Veterans Affairs Primary Care Providers: A National Online Survey. Mil Med. 2020 Mar 2;185(3-4):512-518. doi: 10.1093/milmed/usz291. PMID: 31865375.
Lee SI, Curtis H, Qureshi S, et al. Specialist recommendation for chemoprevention medications in patients at familial risk of breast cancer: a cross-sectional survey in England. J Community Genet. 2021; 12(1):111–20.
Kyalwazi B, Yau C, Campbell MJ, Yoshimatsu TF, Chien AJ, Wallace AM, Forero-Torres A, Pusztai L, Ellis ED, Albain KS, Blaes AH, Haley BB, Boughey JC, Elias AD, Clark AS, Isaacs CJ, Nanda R, Han HS, Yung RL, Tripathy D, Edmiston KK, Viscusi RK, Northfelt DW, Khan QJ, Asare SM, Wilson A, Hirst GL, Lu R, Symmans WF, Yee D, DeMichele AM, van 't Veer LJ, Esserman LJ, Olopade OI. Race, Gene Expression Signatures, and Clinical Outcomes of Patients With High-Risk Early Breast Cancer. JAMA Netw Open. 2023 Dec 1;6(12):e2349646. doi: 10.1001/jamanetworkopen.2023.49646. PMID: 38153734; PMCID: PMC10755617.
Omilian AR, Wei L, Hong CC, Bandera EV, Liu S, Khoury T, Ambrosone CB, Yao S. Somatic mutations of triple-negative breast cancer: a comparison between Black and White women. Breast Cancer Res Treat. 2020 Jul;182(2):503-509. doi: 10.1007/s10549-020-05693-4. Epub 2020 May 21. PMID: 32441016; PMCID: PMC7313393.