
Efficacy of Different Concentrations of Insect Growth Regulators (IGRs) on Maize Stem Borer Infestation
Environmental Sciences Agriculture受け取った 28 Dec 2023 受け入れられた 07 Feb 2024 オンラインで公開された 08 Feb 2024
Focusing on Biology, Medicine and Engineering ISSN: 2995-8067 | Quick Google Scholar
Next Full Text
Modeling of an Electric-fired Brick Oven, Directly Heated
Previous Full Text
The University Sector is Converging on Manufacturing in UK


受け取った 28 Dec 2023 受け入れられた 07 Feb 2024 オンラインで公開された 08 Feb 2024
Maize stem borer (Chilo Partellus), poses a substantial threat to maize crops all over the world, causing damage that ranges from 26.7% to 80.4%. Its destructive impact includes killing the shoot in young plants, leading to a “dead heart,” and damaging the upper stem in older plants through boring activity. To combat this issue, a field experiment evaluated various insect growth regulators (IGRs) such as Lufenuron®, Pyriproxyfen®, Sitara®, Viper®, Track®, and Priority®. These IGRs, known as reduced-risk pesticides, target pest juveniles and are less harmful to beneficial insects. Conducted through a Randomized Complete Block Design (RCBD) with 24 treatments, excluding a control group, the study recorded maize stem borer populations at 3, 7, and 14 days post-IGR application. In conclusion, the study identifies pyriproxyfen® and Priority® at double the standard concentrations as highly effective insect growth regulators in mitigating maize stem borer infestations, offering promising avenues for enhanced pest control strategies in maize cultivation.
Agriculture, serving as the backbone of the economy, employs 50% of the total national labor force and contributes 25% to the GDP, while remarkably accounting for 85% of the country’s export earnings []. Maize (Zea mays L.) serves as a popular fodder crop and is cultivated across an expanse of 967 thousand hectares, generating an annual production of 1731 thousand tons, with an average yield of 1970 kg per hectare in Pakistan [,]. Maize has high nutritive value as it contains 72% starch, 10% protein, 4.80% oil, 9.50% fiber, 3.0% sugar, and 1.70% ash. Because of its high yield among the cereals, it is also known as the “King of the grain crops.” [,]. Spring maize is a success story in agriculture, but the scarcity of quality seed is a major limitation, with only 34% of improved seed available. Market stability suffers due to inadequate drying and grain storage facilities. Notably, maize grain usage in Punjab province for poultry feed has significantly risen from 23% in 2001 to a substantial 55% by 2007 []. However, maize is a pivotal cereal crop globally, contributing significantly to various sectors all over the world. It serves as a versatile crop, providing food, fuel, feed, and fodder for both humans and livestock, while also serving as a source of raw materials for industrial products [], for manufacturing paper making, brewing products like corn sugar, corn oil, corn protein, corn-flacks, and corn syrup etc [,].
Further, there is a large gap between the potential and actual yield of maize due to attacks of insect pests, which are major factors in reducing the yield [,]. Among major insect pests of Rice crops, the Spotted stem borer (Chilo partellus) was recognized as one of the serious pest of maize crop [-]. The young plant of the maize crop can be destroyed and cause the dead heart in plants and in the old plants, this borer results in the upper part of the stem being destroyed [,]. Some contact and systemic insecticides are used for management strategies, which gave the best results against borer attacks [-], but they create resistance in borers and polluting environment []. Recently, for control strategies, some Insect Growth Regulators (IGRs) have been used to stop pest development, reduce their growth, or cause mortality in insect pests [,].
However, recognizing these significant implications, scientists worldwide are redirecting their focus toward developing safer and more sustainable methods for managing insect pests with minimal reliance on toxic substances. They explore eco-friendly control measures to mitigate damage caused by maize borer and shoot flies in maize crops. This research project aimed to assess the effectiveness and residual toxicity of different formulations of insect growth regulators (IGRs) such as Sitara® (buprofezin), Viper® (buprofezin), Lufenuron®, Pyriproxyfen®, Priority® (Pyriproxyfen), and Track® (Lufenuron) against maize stem borer.
The experiment was conducted under field conditions in the year 2011 at the Youngwala experimental area of the Department of Agricultural Entomology, University of Agriculture Faisalabad. The aim was to investigate the efficacy of various formulations of Insect Growth Regulators (IGRs) against maize borer.
A hybrid maize cultivar, AAS-9732 (Pioneer Seed Company) was purchased from the market and cultivated in the research area. The maize variety AAS-9732 hybrid corn obtained from a pioneer seed company was sown in 2011. The Chopra method was used to sow the maize crop. The recommended Agronomic practices were done before sowing the crop. The crop was sown during the normal season of sowing in Randomized Complete Block Design (RCBD), maintaining row-to-row and plant-to-plant distance of 1.5 sq. ft and 0.9 sq. ft, respectively, and plot size was 21780 sq. ft.
The experiment employed various Insect Growth Regulators (IGRs) at different concentrations against maize borer, namely Viper® (buprofezin) 25WP Agri Top, Sitara® (buprofezin) 25WP Ali Akbar, Lufenuron® (Lufenuron®) 5% EC Syngenta, Pyriproxyfen® (pyriproxyfen®) 1.8% EC Kanzo, Priority® (pyriproxyfen) 10.8% EC Kanzo, and Track® (lufenuron). The primary objective of the 2011 study was to assess the effectiveness of diverse formulations of insect growth regulators (Sitara®, Viper®, Priority®, Track®, Lufenuron®, and Pyriproxyfen®) against the maize stem borer (C. partellus) using the hybrid corn variety AAS-9732. Following a Randomized Complete Block Design (RCBD), maize crops were sown during the regular growing season, maintaining specific plant-to-plant (P×P) and row-to-row (R×R) distances. The experimental layout comprised 36 units organized into three blocks, each consisting of 72 units and separated by pathways.
The study involved 25 variations, including a control (T0), established as a baseline for comparative assessments among different concentrations. The control treatment (T0) did not entail the application of any insect growth regulators or insecticides, allowing for a direct evaluation of each treatment’s relative effectiveness compared to others.
The experiment consisted of 25 treatments including the control given in Table 1.
The experimental area consisted of 72 experimental units which were separated into three blocks and isolated by a path of width 24 cm. The twenty-four treatments were completely randomized in each block excluding control, whereas sprays of IGRs were exercised two times during the experimental period at 15-day intervals. The first application was done a few days after germination and then 2nd application was done when the again ETL was recorded.
The data regarding infestation, larvae/plant, and tunnel length inside the stem was recorded throughout the research period at
3, 7, and 14 days post-treatment.
The data collected was subjected to the ANOVA technique for the determination of the level of significance of treatments; whereas the Tukey HSD test was used for the comparison of means if treatment shows significant results.
Influence of Varied IGR Concentrations on Maize Stem Borer Infestation Percentage at 1, 3, 7, and 14 days: The study investigated the impact of different concentrations of insect growth regulators (IGRs) on maize stem borer infestation across varying time intervals. Overall, the treatments exhibited significant effects on infestation levels, with the lowest infestations consistently observed at concentrations equivalent to 2x or 1/2x of the Field Recommended Dose (FRD) for each IGR. Specifically, Sitara®, Viper®, Lufenuron®, Pyriproxyfen®, Priority®, and Track® treatments demonstrated infestation reductions ranging from 0.33% to 9.26% at different concentrations and time points. Notably, infestation levels tended to vary between approximately 2.3% to 9% across various concentrations and post-treatment intervals, indicating differential effectiveness based on concentration and duration after application (Figure 1A-1D).
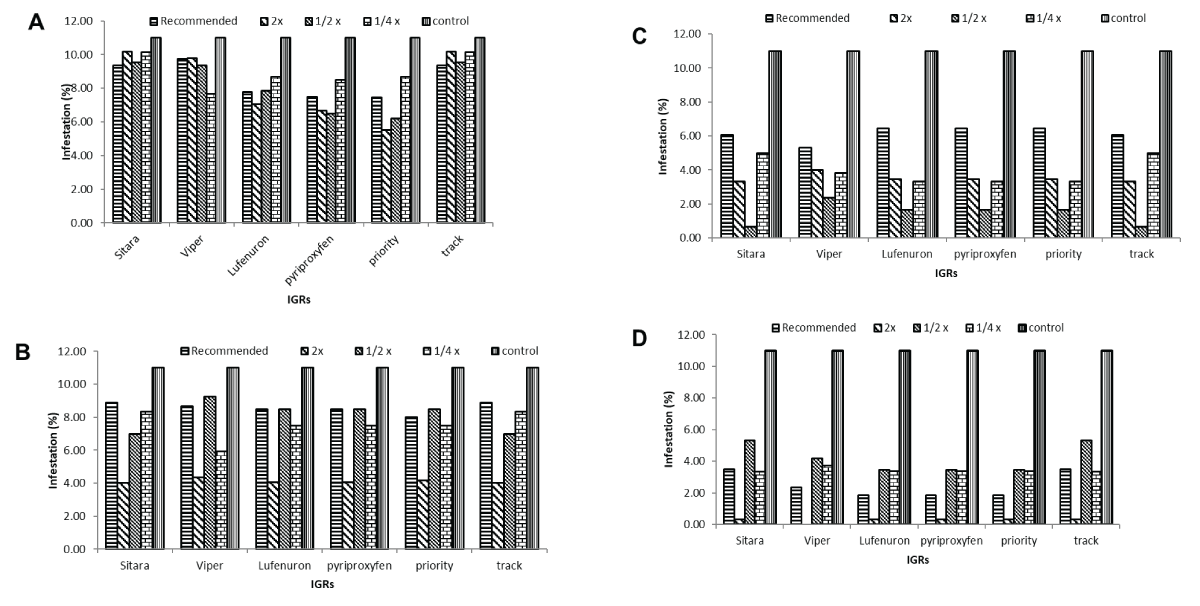 Figure 1: Impact of different concentrations of insect growth regulators (IGRs) on maize stem borer infestation across time intervals. (A) Sitara® concentrations reduced maize stem borer infestation by 0.33% to 9.26% over time. (B) Viper® exhibited fluctuating infestation levels, with reductions between 0.33% and 9.26%. (C) Lufenuron® visually showed reductions from 0.33% to 9.26% across concentrations and time intervals. (D) Pyriproxyfen®, Priority®, and Track® treatments displayed infestation variations from 2.3% to 9%.
Figure 1: Impact of different concentrations of insect growth regulators (IGRs) on maize stem borer infestation across time intervals. (A) Sitara® concentrations reduced maize stem borer infestation by 0.33% to 9.26% over time. (B) Viper® exhibited fluctuating infestation levels, with reductions between 0.33% and 9.26%. (C) Lufenuron® visually showed reductions from 0.33% to 9.26% across concentrations and time intervals. (D) Pyriproxyfen®, Priority®, and Track® treatments displayed infestation variations from 2.3% to 9%.Percentage infestation and reduction rates at various IGR concentrations at 3, 7, and 14 days post-initial application: Different concentrations of FRD showcased varying percentages of infestation and reductions at different post-treatment intervals. At 3 days, infestation ranged from 8.41% in FRD to 4.17% in 2x FRD, demonstrating reductions from 1.29% to 49.27%. Moving to the 7-day interval, infestation varied between 6.12% in FRD to 1.45% in 1/2x FRD, with reductions from 28.16% to 82.20%. Lastly, at 14 days, infestation levels spanned from 3.62% in 1/2x FRD to 0.27% in 2x FRD, depicting reductions from 55.58% to 96.71%. Notably, 2x FRD demonstrated the highest reduction (96.71%) in infestation at the 14-day post-treatment interval (Figure 2A-2C).
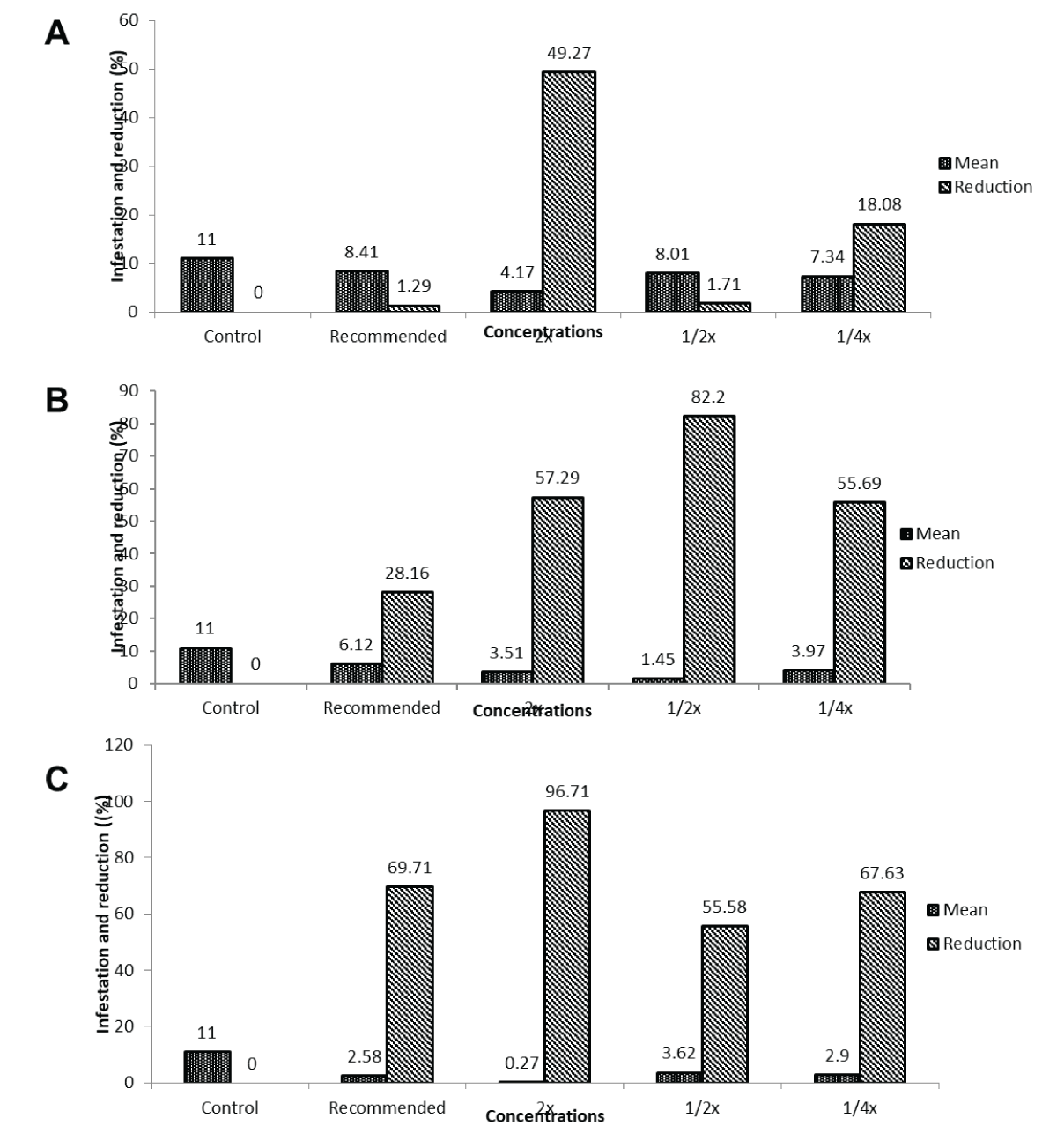 Figure 2: Effect of varying concentrations of field recommended dose (FRD) on maize stem borer infestation across post-treatment intervals. (A) Shows infestation percentages and reductions at different Field Recommended Dose (FRD) concentrations for maize stem borer, with 2x FRD exhibiting the highest reduction of 96.71% at 14 days. (B) Illustrates varied infestation levels and reductions at the 7-day interval, ranging from 28.16% to 82.20% across FRD concentrations. (C) Depicts the infestation trend over different post-treatment intervals, emphasizing 2x FRD’s highest reduction (96.71%) at 14 days for maize stem borer.Second application of IGRs.
Figure 2: Effect of varying concentrations of field recommended dose (FRD) on maize stem borer infestation across post-treatment intervals. (A) Shows infestation percentages and reductions at different Field Recommended Dose (FRD) concentrations for maize stem borer, with 2x FRD exhibiting the highest reduction of 96.71% at 14 days. (B) Illustrates varied infestation levels and reductions at the 7-day interval, ranging from 28.16% to 82.20% across FRD concentrations. (C) Depicts the infestation trend over different post-treatment intervals, emphasizing 2x FRD’s highest reduction (96.71%) at 14 days for maize stem borer.Second application of IGRs.Percent infestation of maize borer 3, 7, and 14 days after the second application and percent reduction in infestation at different IGR concentrations: At various post-treatment intervals, different concentrations of the treatment displayed distinct percentages of infestation and reductions. At 3 days post-treatment, the concentrations of 1/4x, 1/2x, FRD, and 2x of FRD exhibited infestation rates of 7.41%, 7.10%, 7.03%, and 4.05%, respectively, with reductions ranging from 14.78% to 53.39%. At 7 days post-treatment, concentrations showed infestation rates of 2.97% to 5.30%, with reductions spanning from 56.27% to 90.43%. By the 14th day, infestation percentages were reduced from 1.22% to 3.95%, with reductions in infestation ranging from 60.30% to 85.96%. Overall, the concentrations of 1/2x and 2x of FRD demonstrated the highest reductions, reaching 90.43% and 85.96%, respectively, at 7- and 14-day post-treatment intervals (Figure 3A-3C).
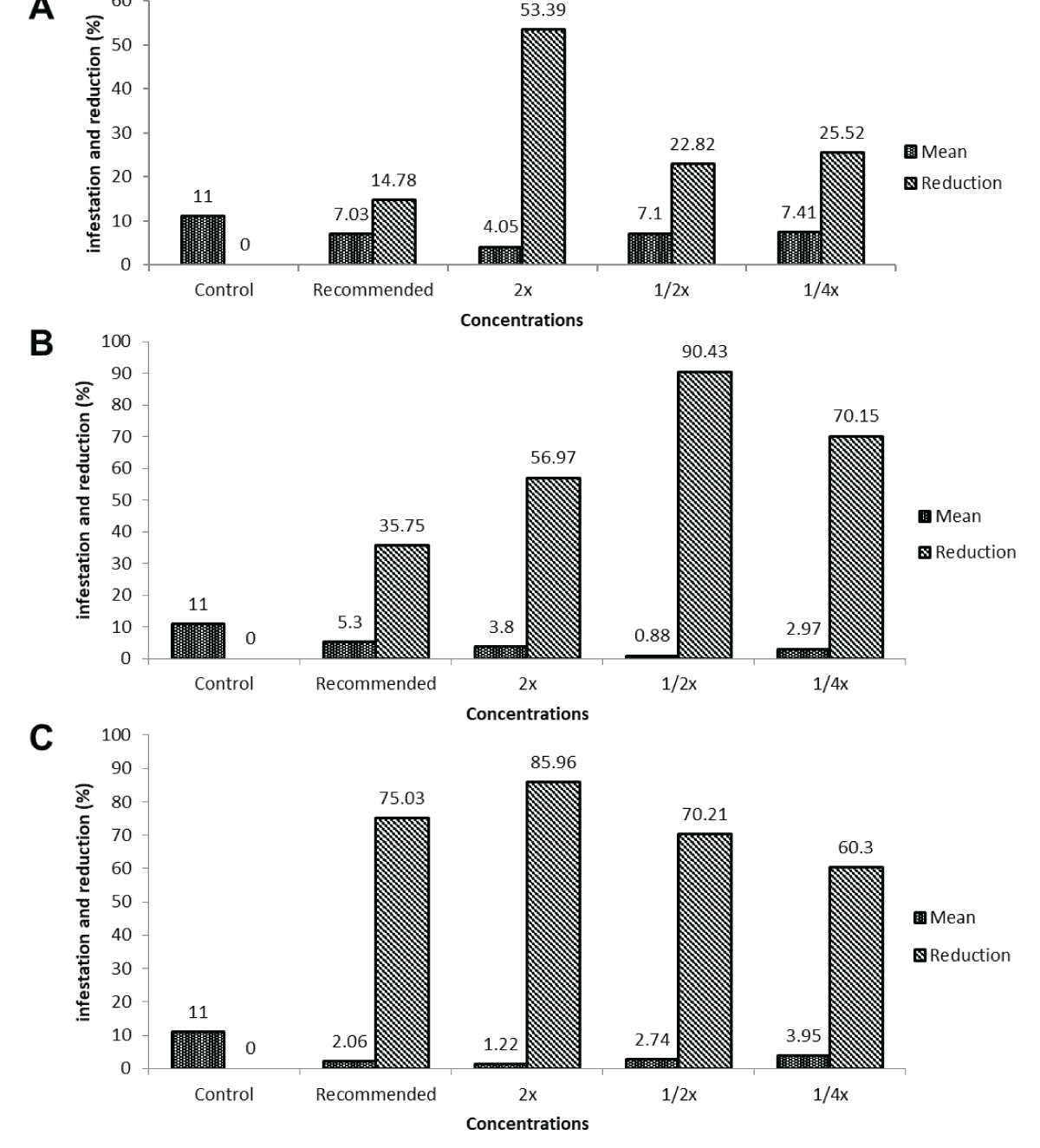 Figure 3: Effect of treatment concentrations on maize stem borer infestation reduction across post-treatment intervals. (A) Shows infestation percentages and reductions at various concentrations, with 1/2x and 2x of FRD exhibiting the highest reductions (53.39%) at 3 days post-treatment for maize stem borer. (B) Illustrates infestation rates and reductions, highlighting that 1/2x and 2x of FRD achieved the highest reductions (90.43%) at 7 days post-treatment. (C) Depicts infestation percentages and reductions over time, emphasizing concentrations of 1/2x and 2x of FRD with the highest reductions (85.96%) at the 14-day post-treatment interval for maize stem borer.
Figure 3: Effect of treatment concentrations on maize stem borer infestation reduction across post-treatment intervals. (A) Shows infestation percentages and reductions at various concentrations, with 1/2x and 2x of FRD exhibiting the highest reductions (53.39%) at 3 days post-treatment for maize stem borer. (B) Illustrates infestation rates and reductions, highlighting that 1/2x and 2x of FRD achieved the highest reductions (90.43%) at 7 days post-treatment. (C) Depicts infestation percentages and reductions over time, emphasizing concentrations of 1/2x and 2x of FRD with the highest reductions (85.96%) at the 14-day post-treatment interval for maize stem borer.
The population of larvae of maize stem borer after the second application
The analysis emphasized the significant impact of treatments in reducing the maize borer population within 24 hours after the second application. Sitara®, Viper®, Lufenuron®, Pyriproxyfen®, Priority®, and Track® treatments revealed diverse larval densities across different concentrations, with lower densities consistently observed at higher concentration levels. Despite the significant impact of treatments, the interaction between insect growth regulators (IGRs) and concentrations did not significantly influence larval density. Notably, concentrations equivalent to 2x Field Recommended Dose (FRD) exhibited the most substantial reduction (64.89%) in larval density compared to other concentrations at the post-treatment interval, indicating the highest effectiveness in reducing larvae per 5 plants (Figure 4A,4B).
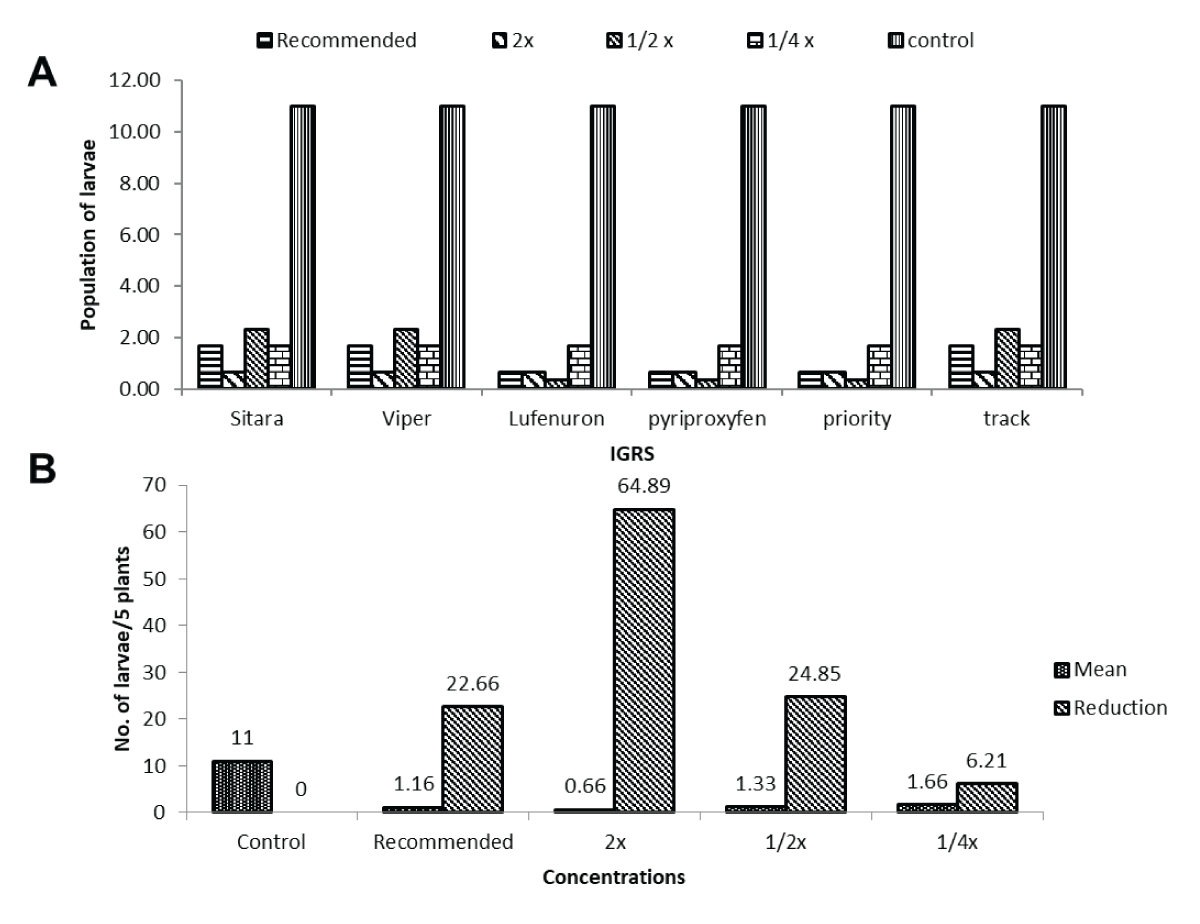 Figure 4: Impact of different concentrations of insect growth regulators (IGRs) on maize borer larval density within 24 hours post second application. (A) Shows varied larval densities with concentrations of Sitara®, Viper®, Lufenuron®, Pyriproxyfen®, Priority®, and Track®, with 2x Field Recommended Dose (FRD) displaying the most substantial reduction (64.89%) within 24 hours after the second application. (B) Emphasizes that despite the significant impact of treatments, the interaction between insect growth regulators (IGRs) and concentrations did not significantly influence larval density, highlighting the efficacy of 2x FRD in achieving the highest reduction (64.89%) in larvae per 5 plants post-treatment.Larval population per five plants of maize borer before and after the second application of different IGRs and its reduction percentage.
Figure 4: Impact of different concentrations of insect growth regulators (IGRs) on maize borer larval density within 24 hours post second application. (A) Shows varied larval densities with concentrations of Sitara®, Viper®, Lufenuron®, Pyriproxyfen®, Priority®, and Track®, with 2x Field Recommended Dose (FRD) displaying the most substantial reduction (64.89%) within 24 hours after the second application. (B) Emphasizes that despite the significant impact of treatments, the interaction between insect growth regulators (IGRs) and concentrations did not significantly influence larval density, highlighting the efficacy of 2x FRD in achieving the highest reduction (64.89%) in larvae per 5 plants post-treatment.Larval population per five plants of maize borer before and after the second application of different IGRs and its reduction percentage.The evaluation post-treatment revealed comparable efficacy among various Insect Growth Regulators (IGRs) in reducing larval density per 5 plants. Viper®, Sitar®, and Track® showed a consistent larval density of 3.46 per 5 plants with varying reduction percentages. Conversely, Lufenuron®, Pyriproxyfen®, and Priority® maintained a similar larval density of 2.86 per 5 plants, coupled with a consistent reduction of 20.55% in larvae per 5 plants. Notably, Lufenuron®, Pyriproxyfen®, and Priority® exhibited the highest reduction rate (20.55%) in larvae per 5 plants at the post-treatment interval. Meanwhile, different concentrations of IGRs showcased varied effects on maize borer tunnel length at the specified post-treatment interval. Varying concentrations of Viper®, Lufenuron®, Pyriproxyfen®, Priority®, and Track® displayed distinct impacts on tunnel length, with notable reductions at concentrations equivalent to 1/2x and 2x of the Field Recommended Dose (FRD), showing substantial reductions of 64.20% and 67.75%, respectively. These findings highlight the effectiveness of specific concentrations in significantly reducing maize borer tunnel lengths post-treatment (Figure 5A-5D).
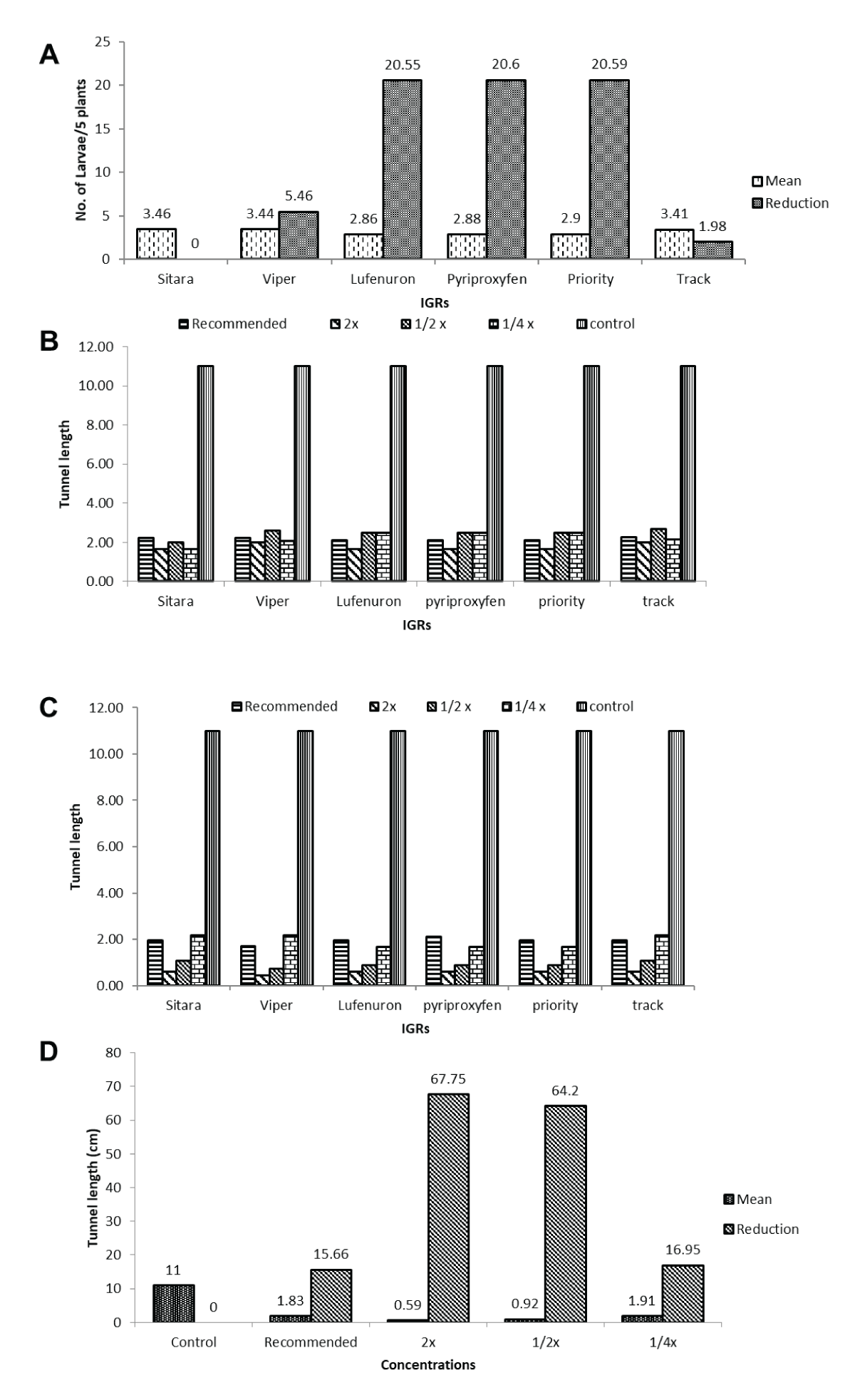 Figure 5: Comparison of insect growth regulators (IGRs) on larval density and tunnel length reductions post-treatment. (A) Shows comparable efficacy of Viper®, Sitara®, and Track® in reducing larval density (3.46 per 5 plants) with varying reduction percentages. (B) Illustrates consistent larval density (2.86 per 5 plants) and the highest reduction rate (20.55%) for Lufenuron®, Pyriproxyfen®, and Priority®. (C) Demonstrates varied effects of concentrations of Viper®, Lufenuron®, Pyriproxyfen®, Priority®, and Track® on maize borer tunnel length, with substantial reductions (64.20% and 67.75%) at 1/2x and 2x FRD equivalents. (D) Highlights the varied impacts of IGR concentrations on maize borer tunnel length, emphasizing specific concentrations’ effectiveness in reducing tunnel lengths post-treatment.
Figure 5: Comparison of insect growth regulators (IGRs) on larval density and tunnel length reductions post-treatment. (A) Shows comparable efficacy of Viper®, Sitara®, and Track® in reducing larval density (3.46 per 5 plants) with varying reduction percentages. (B) Illustrates consistent larval density (2.86 per 5 plants) and the highest reduction rate (20.55%) for Lufenuron®, Pyriproxyfen®, and Priority®. (C) Demonstrates varied effects of concentrations of Viper®, Lufenuron®, Pyriproxyfen®, Priority®, and Track® on maize borer tunnel length, with substantial reductions (64.20% and 67.75%) at 1/2x and 2x FRD equivalents. (D) Highlights the varied impacts of IGR concentrations on maize borer tunnel length, emphasizing specific concentrations’ effectiveness in reducing tunnel lengths post-treatment.Tunnel length (cm) before and after the second application and its percent reduction of different IGRs
At the specified post-treatment interval, various insect growth regulators (IGRs) exhibited comparable effectiveness in reducing tunnel lengths caused by maize borers. Sitar®, Track®, Lufenuron®, Priority®, Viper®, and Pyriproxyfen® demonstrated reductions in tunnel length ranging from 16.20% to 20.75%. Notably, Pyriproxyfen® showcased the highest reduction of 20.75% in tunnel lengths, showcasing its efficacy in mitigating the impact of maize borer tunneling activities at this interval (Figure 6).
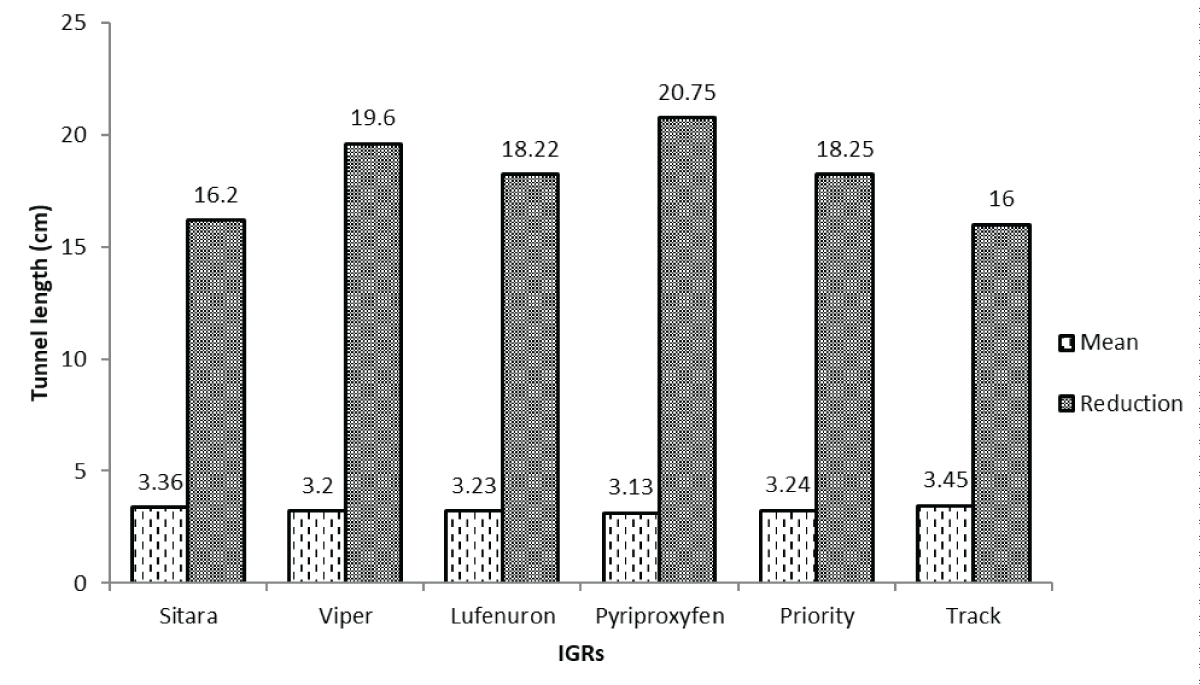 Figure 6: Comparison of insect growth regulators (IGRs) on maize borer tunnel length reductions at specified post-treatment interval. This comparison evaluates how various insect growth regulators (IGRs) impact the reduction of maize borer tunnel length at a specific post-treatment interval, revealing insights into their varying effectiveness in mitigating tunneling damage and emphasizing the potential of specific IGR concentrations to significantly manage this pest.
Figure 6: Comparison of insect growth regulators (IGRs) on maize borer tunnel length reductions at specified post-treatment interval. This comparison evaluates how various insect growth regulators (IGRs) impact the reduction of maize borer tunnel length at a specific post-treatment interval, revealing insights into their varying effectiveness in mitigating tunneling damage and emphasizing the potential of specific IGR concentrations to significantly manage this pest.The study aimed to evaluate the effectiveness of various insect growth regulators (IGRs) against the maize stem borer, pinpointing pyriproxyfen® and priority® as the most potent in reducing infestation. A recent study emphasized the importance of integrating insect growth regulators (IGRs) into a comprehensive IPM strategy for maize crops [-]. This approach ensures a balanced and sustainable pest management system by combining chemical, biological, and cultural control methods. The study’s findings on the effectiveness of pyriproxyfen® and priority® align with the broader consensus on the potential of IGRs in IPM for their targeted action against specific pests [-]. Similarly, ecological studies underscore the importance of assessing the ecological impact of pest control measures []. While IGRs are generally considered less harmful to beneficial insects, the study’s ecological considerations should be interpreted in the context of the latest research on non-target species and overall ecosystem health. This includes investigations into the persistence of IGRs in the environment and potential sublethal effects on non-target organisms [,]. The economic viability of IGRs, including the cost of application and potential yield increase, has been a focal point in recent agricultural economics research [,]. The study’s evaluation of pyriproxyfen® and priority® should be considered in light of the latest economic analyses, exploring the cost-effectiveness of these IGRs compared to traditional pesticides and their impact on overall farm profitability. Recent studies emphasized the need for synergistic approaches in pest management []. Considering the potential interactions between IGRs and other pest control methods, such as biological control or cultural practices, can enhance overall effectiveness and sustainability. The study’s findings should be discussed in the context of the latest research on integrated approaches to pest management, highlighting opportunities for combining IGRs with complementary strategies [-].
In conclusion, the study’s implications extend beyond its immediate findings, intertwining with the latest advancements in agronomic, economic, and environmental research. By incorporating the most recent references, the discussion can offer a comprehensive understanding of the broader implications of the research on maize borer control strategies.
Muhammad Salman Hameed and Yanliang Ren conceived, designed, and wrote the manuscript. Nida Urooj and Khurshied Ahmad Khan helped with table preparation and statistical analysis, while Yanliang Ren and Midori Tuda revised and finalized the manuscripts. All authors approved the submitted version.
This work was supported by the National Key Research and Development Program (2023YFE0110100) and the Natural Science Foundation of China (No. 22377030).
Jatoi FZ. Agriculture in Pakistan and its impact on Economic growth. Available at SSRN 3771914. 2020; 1-21.
Gul H, Rahman S, Shahzad A, Gul S, Qian M, Xiao Q, Liu Z. Maize (Zea mays L.) productivity in response to nitrogen management in Pakistan. American Journal of Plant Sciences. 2021; 12:1173-1179.
Tariq M, Iqbal H. Maize in Pakistan–an overview. Agriculture and Natural Resources. 2010; 44:757-763.
Saeed MS, Saeed A. Health benefits of maize crop-an overview. Current Research in Agriculture and Farming. 2020; 1: 5-8.
Truswell AS, Brock JF. The nutritive value of maize protein for man. Am J Clin Nutr. 1962 Feb; 10:142-52. doi: 10.1093/ajcn/10.2.142. PMID: 13922725.
Ranum P, Peña-Rosas JP, Garcia-Casal MN. Global maize production, utilization, and consumption. Ann N Y Acad Sci. 2014 Apr; 1312:105-12. doi: 10.1111/nyas.12396. Epub 2014 Mar 20. PMID: 24650320.
Dabija A, Ciocan ME, Chetrariu A, Codină GG. Buckwheat and Amaranth as Raw Materials for Brewing, a Review. Plants (Basel). 2022 Mar 12;11(6):756. doi: 10.3390/plants11060756. PMID: 35336638; PMCID: PMC8954860.
Kaur D, Bhardwaj NK, Lohchab RK. Prospects of rice straw as a raw material for paper making. Waste Manag. 2017 Feb; 60:127-139. doi: 10.1016/j.wasman.2016.08.001. Epub 2016 Aug 16. PMID: 27543175.
Chintalapati P, Rathod S, Repalle N, Varma NRG, Karthikeyan K, Sharma S, Kumar RM, Katti G. Insect Pest Incidence with the System of Rice Intensification: Results of a Multi-Location Study and a Meta-Analysis. Agronomy. 2023; 13:1-15.
Gupta S, Handore K, Pandey I. Effect of insecticides against Chilo partellus (Swinhoe) damaging Zea mays (maize). International Journal of Parasitology Research. 2010; 2: 1-7.
Achiri TD, Atakan E, Pehlivan S. Invasive maize spotted stem borer Chilo partellus Swinhoe 1885 (Lepidoptera: Crambidae) is the dominant pest in maize agro-system in the Mediterranean Region of Turkey. Journal of Asia-Pacific Entomology. 2022; 25:1-9.
Leonard A, Rwegasira GM. Abundance and Spatial Dispersion of Rice Stem Borer Species in Kahama, Tanzania. J Insect Sci. 2015 Sep 27;15(1):132. doi: 10.1093/jisesa/iev106. PMID: 26411785; PMCID: PMC4664944.
Van den Berg J, Van Rensburg J, Pringle K. Damage caused by Chilo partellus (Swinhoe)(Lepidoptera: Pyralidae) to various cultivars of grain sorghum, Sorghum bicolor (L.) Moench. South African Journal of Plant and Soil. 1990; 7: 192-196.
Horgan FG, Romena AM, Bernal CC, Almazan MLP, Ramal AF. Stem borers revisited: Host resistance, tolerance, and vulnerability determine levels of field damage from a complex of Asian rice stemborers. Crop Prot. 2021 Apr; 142:105513. doi: 10.1016/j.cropro.2020.105513. PMID: 33814663; PMCID: PMC7846815.
January B, Rwegasira G, Tefera F. Lepidopteran stem borer species abundance and associated damages on irrigated Kilombero low land rice ecosystem in Tanzania. Journal of Entomology. 2018; 1-8.
Katel S, Lamshal BS, Singh Yadav SP, Timsina S, Mandal HR, Kattel S, Adhikari S, Adhikari N. Efficacy of different insecticides against the yellow stem borer (Scirpophaga incertulus Walker) (Lepidoptera: Crambidae) in spring rice cultivation. Cogent Food & Agriculture. 2023; 9:1-11.
Sardar MU, Ali S, Mamoon-ur-Rashid M, Naeem M, Saad M, Khan AS. Evaluation Of Synthetic Insecticides For The Control Of Maize Stem Borer, Chilo Partellus (Swinhoe) (Crambidae: Lepidoptera) In DI Khan, Khyber Pakhtunkhwa, Pakistan. NVEO-Natural Volatiles & Essential Oils. 2022; 1823-1836.
Teli V, Chavan B, Ankalkoppe M, Khot R, Harers P. Evaluation of some insecticides for the control of maize stem borer, Chilo partellus (Swinhoe). Journal of Entomological Research. 2007; 31:323-326.
Patel C, Patel C, Varma C. Bio-chemical basis of resistance to stem borer, Chilo partellus (Swinhoe) infesting forage sorghum, Sorghum bicolour (L.) Moench. Journal of Entomology and Zoology Studies. 2021; 9: 834-839.
Masih SC, Ahmad BR. Insect growth regulators for insect pest control. International Journal of Current Microbiology and Applied Sciences. 2019; 8: 208-218.
Tunaz H, Uygun N. Insect growth regulators for insect pest control. Turkish Journal of Agriculture and Forestry. 2004; 28: 377-387.
Anderson JA, Ellsworth PC, Faria JC, Head GP, Owen MDK, Pilcher CD, Shelton AM, Meissle M. Genetically Engineered Crops: Importance of Diversified Integrated Pest Management for Agricultural Sustainability. Front Bioeng Biotechnol. 2019 Feb 20; 7:24. doi: 10.3389/fbioe.2019.00024. PMID: 30842944; PMCID: PMC6391707.
Torres JB, Bueno AdF. Conservation biological control using selective insecticides–a valuable tool for IPM. Biological Control. 2018; 126: 53-64.
Veres A, Wyckhuys KAG, Kiss J, Tóth F, Burgio G, Pons X, Avilla C, Vidal S, Razinger J, Bazok R, Matyjaszczyk E, Milosavljević I, Le XV, Zhou W, Zhu ZR, Tarno H, Hadi B, Lundgren J, Bonmatin JM, van Lexmond MB, Aebi A, Rauf A, Furlan L. An update of the Worldwide Integrated Assessment (WIA) on systemic pesticides. Part 4: Alternatives in major cropping systems. Environ Sci Pollut Res Int. 2020 Aug;27(24):29867-29899. doi: 10.1007/s11356-020-09279-x. Epub 2020 Jun 4. PMID: 32500500; PMCID: PMC7378116.
Achee NL, Grieco JP, Vatandoost H, Seixas G, Pinto J, Ching-Ng L, Martins AJ, Juntarajumnong W, Corbel V, Gouagna C, David JP, Logan JG, Orsborne J, Marois E, Devine GJ, Vontas J. Alternative strategies for mosquito-borne arbovirus control. PLoS Negl Trop Dis. 2019 Jan 3;13(1):e0006822. doi: 10.1371/journal.pntd.0006822. Erratum in: PLoS Negl Trop Dis. 2019 Mar 26;13(3):e0007275. PMID: 30605475; PMCID: PMC6317787.
Nyirakanani C. Analysis of Cassava Brown Streak Disease in Rwanda: Incidence, Dissemination, Genetic Diversity, and Innovative Mitigation Strategies. French Community of Belgium University of Liege _ Gembloux Agro-Bio Tech. 2023; 1-174.
Wickliffe LC. Decision-Support Tool for Residential Pesticides in the South Carolina Coastal Zone (University of South Carolina).
Rodríguez-San Pedro A, Allendes JL, Beltrán CA, Chaperon PN, Saldarriaga-Córdoba MM, Silva AX, Grez AA. Quantifying ecological and economic value of pest control services provided by bats in a vineyard landscape of central Chile. Agriculture, Ecosystems & Environment. 2020; 302:1-9.
Liégeois S, Delaunay M, Lécureuil C, Goubault M. Sublethal doses of pyriproxyfen stimulate reproduction and aggressive behavior in a non-target parasitoid wasp. Sci Total Environ. 2022 Oct 10; 842:156880. doi: 10.1016/j.scitotenv.2022.156880. Epub 2022 Jun 23. PMID: 35753446.
Sánchez-Bayo F. Insecticides mode of action in relation to their toxicity to non-target organisms. Journal of Environmental and Analytical Toxicology. 2012; 4:1-9.
Chavan S, Dhillon R, Sirohi C, Keerthika A, Kumari S, Bharadwaj K, Jinger D, Kakade V, Chichaghare A, Zin El-Abedin TK. Enhancing farm income through boundary plantation of poplar (Populus deltoides): An economic analysis. Sustainability. 2022; 14: 1-17.
Curk M, Trdan S. Unlocking Synergistic Potentials: Exploring Enhanced Biological Control Efficacy Through Simultaneous Use of Various Methods for Combating Pest Pressure in Agriculture. Preprints. 2023; 1-9.
Arthur FH, Ghimire MN, Myers SW, Phillips TW. Evaluation of Pyrethroid Insecticides and Insect Growth Regulators Applied to Different Surfaces for Control of Trogoderma granarium (Coleoptera: Dermestidae) the Khapra Beetle. J Econ Entomol. 2018 Apr 2;111(2):612-619. doi: 10.1093/jee/toy040. PMID: 29514245.
Arthur FH, Liu S, Zhao B, Phillips TW. Residual efficacy of pyriproxyfen and hydroprene applied to wood, metal and concrete for control of stored-product insects. Pest Manag Sci. 2009 Jul;65(7):791-7. doi: 10.1002/ps.1756. PMID: 19360716.
Athanassiou CG, Arthur FH, Kavallieratos NG, Throne JE. Efficacy of pyriproxyfen for control of stored-product psocids (Psocoptera) on concrete surfaces. J Econ Entomol. 2011 Oct;104(5):1765-9. doi: 10.1603/ec10424. PMID: 22066208.
Cloyd RA. Effect of insect growth regulators on citrus mealybug [Planococcus citri (Homoptera: Pseudococcidae)] egg production. HortScience. 2003; 38:1397-1399.
Gogi MD, Sarfraz RM, Dosdall LM, Arif MJ, Keddie AB, Ashfaq M. Effectiveness of two insect growth regulators against Bemisia tabaci (Gennadius) (Homoptera: Aleyrodidae) and Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) and their impact on population densities of arthropod predators in cotton in Pakistan. Pest Manag Sci. 2006 Oct;62(10):982-90. doi: 10.1002/ps.1273. PMID: 16862616.
He F, Sun S, Sun X, Ji S, Li X, Zhang J, Jiang X. Effects of insect growth-regulator insecticides on the immature stages of Harmonia axyridis (Coleoptera: Coccinellidae). Ecotoxicol Environ Saf. 2018 Nov 30; 164:665-674. doi: 10.1016/j.ecoenv.2018.08.076. Epub 2018 Aug 28. Erratum in: Ecotoxicol Environ Saf. 2020 Feb; 189:109950. PMID: 30170315.
James DG. Effect of buprofezin on survival of immature stages of Harmonia axyridis, Stethorus punctum picipes (Coleoptera: Coccinellidae), Orius tristicolor (Hemiptera: Anthocoridae), and Geocoris spp. (Hemiptera: Geocoridae). J Econ Entomol. 2004 Jun;97(3):900-4. doi: 10.1093/jee/97.3.900. PMID: 15279269.
Liu T-X, Stansly PA. Lethal and sublethal effects of two insect growth regulators on adult Delphastus catalinae (Coleoptera: Coccinellidae), a predator of whiteflies (Homoptera: Aleyrodidae). Biological Control. 2004; 30: 298-305.
Mashwani A, Ullah F, Sattar S, Ahmand S, Khan M. Efficacy of different insecticides against maize stem borer, Chilo partellus swinhoe (Lepidoptera: Pyralidae) at Peshawar and Swat valleys of Khyber Pakhutunkhwa, Pakistan. Sarhad Journal of Agriculture. 2011; 27:459-465.
Reddy GV, Kumar A. Efficacy of various insecticides against maize stem borer Chilo partellus (Swinhoe) and their cost-benefit analysis at Prayagraj. Journal of Entomology and Zoology Studies. 2021; 9:130-134.
Siddall JB. Insect growth regulators and insect control: a critical appraisal. Environ Health Perspect. 1976 Apr; 14:119-26. doi: 10.1289/ehp.7614119. PMID: 976222; PMCID: PMC1475088.
Suhail A, Arif MJ, Yazdani MS. Comparative efficacy of some insecticides against insect pest complex of maize. Pakistan Journal of Biological Sciences (Pakistan). 2000; 3:1052-1053.
Hameed MS, Khan KA, Urooj N, Noorka IR. Efficacy of Different Concentrations of Insect Growth Regulators (IGRs) on Maize Stem Borer Infestation. IgMin Res. Feb 08, 2024; 2(2): 066-072. IgMin ID: igmin147; DOI: 10.61927/igmin147; Available at: www.igminresearch.com/articles/pdf/igmin147.pdf
次のリンクを共有した人は、このコンテンツを読むことができます:
1National Key Laboratory of Green Pesticide, International Joint Research Center for Intelligent Biosensor Technology and Health, Central China Normal University, Wuhan 430079, P.R. China
2HATO Agriculture Lighting, National Science and Technology Park (NSTP) NUST, G405-406, Islamabad, Pakistan
3Department of Business Administration, Bahaudin Zakriya University, Multan, Pakistan
4Department of Plant Breeding and Genetics University of Sargodha, Pakistan
Address Correspondence:
Muhammad Salman Hameed, National Key Laboratory of Green Pesticide, International Joint Research Center for Intelligent Biosensor Technology and Health, Central China Normal University, Wuhan 430079, P.R. China
How to cite this article:
Hameed MS, Khan KA, Urooj N, Noorka IR. Efficacy of Different Concentrations of Insect Growth Regulators (IGRs) on Maize Stem Borer Infestation.
IgMin Res. Feb 08, 2024; 2(2): 066-072. IgMin ID: igmin147; DOI: 10.61927/igmin147; Available at: www.igminresearch.com/articles/pdf/igmin147.pdf
Copyright: © 2024 Hameed MS, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
 Figure 1: Impact of different concentrations of insect growt...
Figure 1: Impact of different concentrations of insect growt...
 Figure 2: Effect of varying concentrations of field recommen...
Figure 2: Effect of varying concentrations of field recommen...
 Figure 3: Effect of treatment concentrations on maize stem b...
Figure 3: Effect of treatment concentrations on maize stem b...
 Figure 4: Impact of different concentrations of insect growt...
Figure 4: Impact of different concentrations of insect growt...
 Figure 5: Comparison of insect growth regulators (IGRs) on l...
Figure 5: Comparison of insect growth regulators (IGRs) on l...
 Figure 6: Comparison of insect growth regulators (IGRs) on m...
Figure 6: Comparison of insect growth regulators (IGRs) on m...
 Table 1: The treatments of insecticides used for the experi...
Table 1: The treatments of insecticides used for the experi...
Jatoi FZ. Agriculture in Pakistan and its impact on Economic growth. Available at SSRN 3771914. 2020; 1-21.
Gul H, Rahman S, Shahzad A, Gul S, Qian M, Xiao Q, Liu Z. Maize (Zea mays L.) productivity in response to nitrogen management in Pakistan. American Journal of Plant Sciences. 2021; 12:1173-1179.
Tariq M, Iqbal H. Maize in Pakistan–an overview. Agriculture and Natural Resources. 2010; 44:757-763.
Saeed MS, Saeed A. Health benefits of maize crop-an overview. Current Research in Agriculture and Farming. 2020; 1: 5-8.
Truswell AS, Brock JF. The nutritive value of maize protein for man. Am J Clin Nutr. 1962 Feb; 10:142-52. doi: 10.1093/ajcn/10.2.142. PMID: 13922725.
Ranum P, Peña-Rosas JP, Garcia-Casal MN. Global maize production, utilization, and consumption. Ann N Y Acad Sci. 2014 Apr; 1312:105-12. doi: 10.1111/nyas.12396. Epub 2014 Mar 20. PMID: 24650320.
Dabija A, Ciocan ME, Chetrariu A, Codină GG. Buckwheat and Amaranth as Raw Materials for Brewing, a Review. Plants (Basel). 2022 Mar 12;11(6):756. doi: 10.3390/plants11060756. PMID: 35336638; PMCID: PMC8954860.
Kaur D, Bhardwaj NK, Lohchab RK. Prospects of rice straw as a raw material for paper making. Waste Manag. 2017 Feb; 60:127-139. doi: 10.1016/j.wasman.2016.08.001. Epub 2016 Aug 16. PMID: 27543175.
Chintalapati P, Rathod S, Repalle N, Varma NRG, Karthikeyan K, Sharma S, Kumar RM, Katti G. Insect Pest Incidence with the System of Rice Intensification: Results of a Multi-Location Study and a Meta-Analysis. Agronomy. 2023; 13:1-15.
Gupta S, Handore K, Pandey I. Effect of insecticides against Chilo partellus (Swinhoe) damaging Zea mays (maize). International Journal of Parasitology Research. 2010; 2: 1-7.
Achiri TD, Atakan E, Pehlivan S. Invasive maize spotted stem borer Chilo partellus Swinhoe 1885 (Lepidoptera: Crambidae) is the dominant pest in maize agro-system in the Mediterranean Region of Turkey. Journal of Asia-Pacific Entomology. 2022; 25:1-9.
Leonard A, Rwegasira GM. Abundance and Spatial Dispersion of Rice Stem Borer Species in Kahama, Tanzania. J Insect Sci. 2015 Sep 27;15(1):132. doi: 10.1093/jisesa/iev106. PMID: 26411785; PMCID: PMC4664944.
Van den Berg J, Van Rensburg J, Pringle K. Damage caused by Chilo partellus (Swinhoe)(Lepidoptera: Pyralidae) to various cultivars of grain sorghum, Sorghum bicolor (L.) Moench. South African Journal of Plant and Soil. 1990; 7: 192-196.
Horgan FG, Romena AM, Bernal CC, Almazan MLP, Ramal AF. Stem borers revisited: Host resistance, tolerance, and vulnerability determine levels of field damage from a complex of Asian rice stemborers. Crop Prot. 2021 Apr; 142:105513. doi: 10.1016/j.cropro.2020.105513. PMID: 33814663; PMCID: PMC7846815.
January B, Rwegasira G, Tefera F. Lepidopteran stem borer species abundance and associated damages on irrigated Kilombero low land rice ecosystem in Tanzania. Journal of Entomology. 2018; 1-8.
Katel S, Lamshal BS, Singh Yadav SP, Timsina S, Mandal HR, Kattel S, Adhikari S, Adhikari N. Efficacy of different insecticides against the yellow stem borer (Scirpophaga incertulus Walker) (Lepidoptera: Crambidae) in spring rice cultivation. Cogent Food & Agriculture. 2023; 9:1-11.
Sardar MU, Ali S, Mamoon-ur-Rashid M, Naeem M, Saad M, Khan AS. Evaluation Of Synthetic Insecticides For The Control Of Maize Stem Borer, Chilo Partellus (Swinhoe) (Crambidae: Lepidoptera) In DI Khan, Khyber Pakhtunkhwa, Pakistan. NVEO-Natural Volatiles & Essential Oils. 2022; 1823-1836.
Teli V, Chavan B, Ankalkoppe M, Khot R, Harers P. Evaluation of some insecticides for the control of maize stem borer, Chilo partellus (Swinhoe). Journal of Entomological Research. 2007; 31:323-326.
Patel C, Patel C, Varma C. Bio-chemical basis of resistance to stem borer, Chilo partellus (Swinhoe) infesting forage sorghum, Sorghum bicolour (L.) Moench. Journal of Entomology and Zoology Studies. 2021; 9: 834-839.
Masih SC, Ahmad BR. Insect growth regulators for insect pest control. International Journal of Current Microbiology and Applied Sciences. 2019; 8: 208-218.
Tunaz H, Uygun N. Insect growth regulators for insect pest control. Turkish Journal of Agriculture and Forestry. 2004; 28: 377-387.
Anderson JA, Ellsworth PC, Faria JC, Head GP, Owen MDK, Pilcher CD, Shelton AM, Meissle M. Genetically Engineered Crops: Importance of Diversified Integrated Pest Management for Agricultural Sustainability. Front Bioeng Biotechnol. 2019 Feb 20; 7:24. doi: 10.3389/fbioe.2019.00024. PMID: 30842944; PMCID: PMC6391707.
Torres JB, Bueno AdF. Conservation biological control using selective insecticides–a valuable tool for IPM. Biological Control. 2018; 126: 53-64.
Veres A, Wyckhuys KAG, Kiss J, Tóth F, Burgio G, Pons X, Avilla C, Vidal S, Razinger J, Bazok R, Matyjaszczyk E, Milosavljević I, Le XV, Zhou W, Zhu ZR, Tarno H, Hadi B, Lundgren J, Bonmatin JM, van Lexmond MB, Aebi A, Rauf A, Furlan L. An update of the Worldwide Integrated Assessment (WIA) on systemic pesticides. Part 4: Alternatives in major cropping systems. Environ Sci Pollut Res Int. 2020 Aug;27(24):29867-29899. doi: 10.1007/s11356-020-09279-x. Epub 2020 Jun 4. PMID: 32500500; PMCID: PMC7378116.
Achee NL, Grieco JP, Vatandoost H, Seixas G, Pinto J, Ching-Ng L, Martins AJ, Juntarajumnong W, Corbel V, Gouagna C, David JP, Logan JG, Orsborne J, Marois E, Devine GJ, Vontas J. Alternative strategies for mosquito-borne arbovirus control. PLoS Negl Trop Dis. 2019 Jan 3;13(1):e0006822. doi: 10.1371/journal.pntd.0006822. Erratum in: PLoS Negl Trop Dis. 2019 Mar 26;13(3):e0007275. PMID: 30605475; PMCID: PMC6317787.
Nyirakanani C. Analysis of Cassava Brown Streak Disease in Rwanda: Incidence, Dissemination, Genetic Diversity, and Innovative Mitigation Strategies. French Community of Belgium University of Liege _ Gembloux Agro-Bio Tech. 2023; 1-174.
Wickliffe LC. Decision-Support Tool for Residential Pesticides in the South Carolina Coastal Zone (University of South Carolina).
Rodríguez-San Pedro A, Allendes JL, Beltrán CA, Chaperon PN, Saldarriaga-Córdoba MM, Silva AX, Grez AA. Quantifying ecological and economic value of pest control services provided by bats in a vineyard landscape of central Chile. Agriculture, Ecosystems & Environment. 2020; 302:1-9.
Liégeois S, Delaunay M, Lécureuil C, Goubault M. Sublethal doses of pyriproxyfen stimulate reproduction and aggressive behavior in a non-target parasitoid wasp. Sci Total Environ. 2022 Oct 10; 842:156880. doi: 10.1016/j.scitotenv.2022.156880. Epub 2022 Jun 23. PMID: 35753446.
Sánchez-Bayo F. Insecticides mode of action in relation to their toxicity to non-target organisms. Journal of Environmental and Analytical Toxicology. 2012; 4:1-9.
Chavan S, Dhillon R, Sirohi C, Keerthika A, Kumari S, Bharadwaj K, Jinger D, Kakade V, Chichaghare A, Zin El-Abedin TK. Enhancing farm income through boundary plantation of poplar (Populus deltoides): An economic analysis. Sustainability. 2022; 14: 1-17.
Curk M, Trdan S. Unlocking Synergistic Potentials: Exploring Enhanced Biological Control Efficacy Through Simultaneous Use of Various Methods for Combating Pest Pressure in Agriculture. Preprints. 2023; 1-9.
Arthur FH, Ghimire MN, Myers SW, Phillips TW. Evaluation of Pyrethroid Insecticides and Insect Growth Regulators Applied to Different Surfaces for Control of Trogoderma granarium (Coleoptera: Dermestidae) the Khapra Beetle. J Econ Entomol. 2018 Apr 2;111(2):612-619. doi: 10.1093/jee/toy040. PMID: 29514245.
Arthur FH, Liu S, Zhao B, Phillips TW. Residual efficacy of pyriproxyfen and hydroprene applied to wood, metal and concrete for control of stored-product insects. Pest Manag Sci. 2009 Jul;65(7):791-7. doi: 10.1002/ps.1756. PMID: 19360716.
Athanassiou CG, Arthur FH, Kavallieratos NG, Throne JE. Efficacy of pyriproxyfen for control of stored-product psocids (Psocoptera) on concrete surfaces. J Econ Entomol. 2011 Oct;104(5):1765-9. doi: 10.1603/ec10424. PMID: 22066208.
Cloyd RA. Effect of insect growth regulators on citrus mealybug [Planococcus citri (Homoptera: Pseudococcidae)] egg production. HortScience. 2003; 38:1397-1399.
Gogi MD, Sarfraz RM, Dosdall LM, Arif MJ, Keddie AB, Ashfaq M. Effectiveness of two insect growth regulators against Bemisia tabaci (Gennadius) (Homoptera: Aleyrodidae) and Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) and their impact on population densities of arthropod predators in cotton in Pakistan. Pest Manag Sci. 2006 Oct;62(10):982-90. doi: 10.1002/ps.1273. PMID: 16862616.
He F, Sun S, Sun X, Ji S, Li X, Zhang J, Jiang X. Effects of insect growth-regulator insecticides on the immature stages of Harmonia axyridis (Coleoptera: Coccinellidae). Ecotoxicol Environ Saf. 2018 Nov 30; 164:665-674. doi: 10.1016/j.ecoenv.2018.08.076. Epub 2018 Aug 28. Erratum in: Ecotoxicol Environ Saf. 2020 Feb; 189:109950. PMID: 30170315.
James DG. Effect of buprofezin on survival of immature stages of Harmonia axyridis, Stethorus punctum picipes (Coleoptera: Coccinellidae), Orius tristicolor (Hemiptera: Anthocoridae), and Geocoris spp. (Hemiptera: Geocoridae). J Econ Entomol. 2004 Jun;97(3):900-4. doi: 10.1093/jee/97.3.900. PMID: 15279269.
Liu T-X, Stansly PA. Lethal and sublethal effects of two insect growth regulators on adult Delphastus catalinae (Coleoptera: Coccinellidae), a predator of whiteflies (Homoptera: Aleyrodidae). Biological Control. 2004; 30: 298-305.
Mashwani A, Ullah F, Sattar S, Ahmand S, Khan M. Efficacy of different insecticides against maize stem borer, Chilo partellus swinhoe (Lepidoptera: Pyralidae) at Peshawar and Swat valleys of Khyber Pakhutunkhwa, Pakistan. Sarhad Journal of Agriculture. 2011; 27:459-465.
Reddy GV, Kumar A. Efficacy of various insecticides against maize stem borer Chilo partellus (Swinhoe) and their cost-benefit analysis at Prayagraj. Journal of Entomology and Zoology Studies. 2021; 9:130-134.
Siddall JB. Insect growth regulators and insect control: a critical appraisal. Environ Health Perspect. 1976 Apr; 14:119-26. doi: 10.1289/ehp.7614119. PMID: 976222; PMCID: PMC1475088.
Suhail A, Arif MJ, Yazdani MS. Comparative efficacy of some insecticides against insect pest complex of maize. Pakistan Journal of Biological Sciences (Pakistan). 2000; 3:1052-1053.