
Association and New Therapy Perspectives in Post-Stroke Aphasia with Hand Motor Dysfunction
Neurology RehabilitationClinical Medicine受け取った 09 Jan 2024 受け入れられた 24 Jan 2024 オンラインで公開された 25 Jan 2024
Focusing on Biology, Medicine and Engineering ISSN: 2995-8067 | Quick Google Scholar


受け取った 09 Jan 2024 受け入れられた 24 Jan 2024 オンラインで公開された 25 Jan 2024
Post-stroke aphasia and hand movement dysfunction are common and disabling conditions. Observations indicate that most patients with post-stroke aphasia also suffer from hand movement dysfunction. Research in human evolution, behavior, and neuroscience has revealed a strong connection between language function and hand-motor function, with the latter playing a critical role in language use. Consequently, there is an urgent need for the development of new, comprehensive, and efficient rehabilitation methods for post-stroke aphasia that is accompanied by hand dysfunction. One promising approach involves investigating the shared neural networks between language and hand function as a foundation for novel treatment methods. This article aims to review the current state of clinical research on comprehensive treatments for stroke-induced aphasia and hand dysfunction, as well as to explore their underlying neural mechanisms. The results of this study may provide a valuable reference for the advancement of treatment technologies that effectively address both dysfunctions and enhance clinical outcomes.
PSA (Post-Stroke Aphasia, PSA) is a common complication in patients with stroke []. More than 90% of stroke survivors experience two or more functional dysfunctions simultaneously, with approximately 24% suffering from both PSA and hemiplegia, which significantly impacts their quality of life []. From the perspective of cerebral anatomy, it is easy to understand that the middle cerebral artery is the thickest branch of the internal carotid artery []. Its branches are widely distributed in the internal capsule and basal ganglia, superior temporal gyrus, middle temporal gyrus, and so on, involving the motor area, language center, and somatosensory area, so stroke patients are often complicated with aphasia and hand dysfunction []. However, with the development of neuroscience and brain imaging technology, the relationship between language and hand function is not limited to the proximity of anatomical position, and the relationship between language and hand motor function far exceeds the relationship of blood supply [-]. From the archaeological discovery of “the representative language form of early murals coinciding with the increased use of hand tools” [], to the study of neuroimaging and electromyography [,], all show that language and hand motor function are interrelated; observation-execution of hand movements can activate the mirror neuron system of the human brain and improve language function to some extent []. Therefore, for stroke patients with aphasia and hand dysfunction, we can explore an efficient and cooperative comprehensive treatment combined with their correlation in behavior and neurology. However, at present, little is known about the potential interaction between cross-modal treatments and we can observe from Figure 1 that the studies of PSA patients with hand motor dysfunction are much lower than those of patients with aphasia or hand motor dysfunction alone and, from an epidemiological point of view, are poorly matched with its prevalence. Non-invasive brain stimulation techniques do not have evidence-based evidence to determine the intervention targets and doses for the coexistence of the two kinds of dysfunction, so there is still a lot of room for exploration and development. Our review mainly focuses on the comprehensive rehabilitation treatment of stroke patients with aphasia and hand motor dysfunction, and combs its neural mechanism, to provide a theoretical basis for the formulation of rehabilitation programs suitable for patients with both dysfunction and optimized clinical treatment programs.
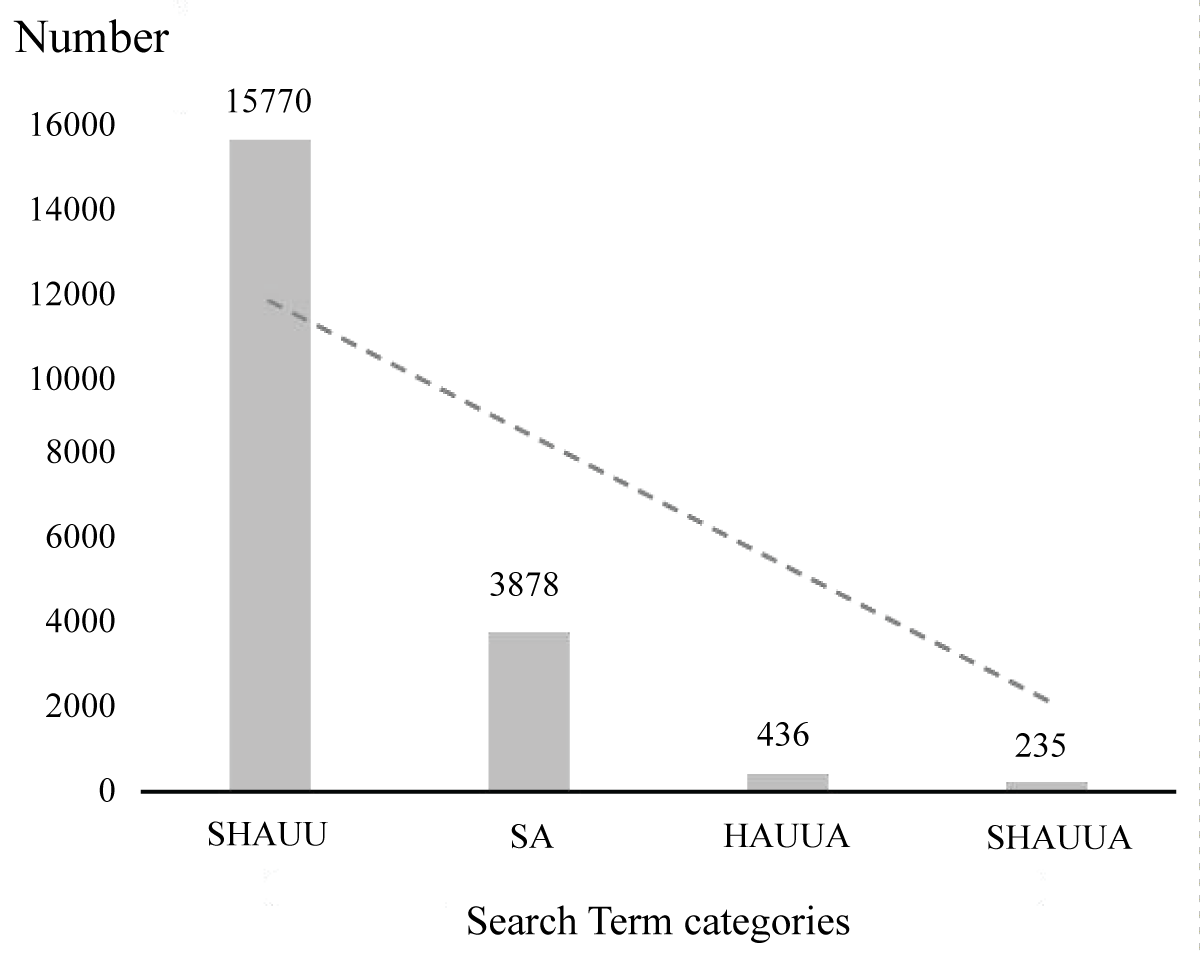 Figure 1: Number of literature searches for keywords related to post-stroke aphasia and hand movement. Abbreviation: SHAUU, (stroke) AND ((hand) OR (arm) OR (upper limb) OR (upper extremity)); SA, aphasia and stroke; HAUUA, ((hand) OR (arm) OR (upper limb) OR (upper extremity)) and (aphasia); SHAUUA, ((hand) OR (arm) OR (upper limb) OR (upper extremity)) and (aphasia) and (stroke). Searched in PUBMED.
Figure 1: Number of literature searches for keywords related to post-stroke aphasia and hand movement. Abbreviation: SHAUU, (stroke) AND ((hand) OR (arm) OR (upper limb) OR (upper extremity)); SA, aphasia and stroke; HAUUA, ((hand) OR (arm) OR (upper limb) OR (upper extremity)) and (aphasia); SHAUUA, ((hand) OR (arm) OR (upper limb) OR (upper extremity)) and (aphasia) and (stroke). Searched in PUBMED.The “gestural theory” of language holds that grasping movement is the origin of speech []. Neurons in the ventrolateral premotor cortex (F5 area) [,], namely Mirror Neurons (MNS), can be excited when macaques perform or observe other individuals (monkeys or humans) perform grasping actions. Brain imaging and other studies have shown that BA44 in the Broca area of the human cerebral cortex has homology with the F5 area of the monkey brain []. Mirror neurons are found in various brain regions of the human brain, particularly the posterior inferior frontal gyrus and premotor cortex, and their activation is crucial for action observation, action understanding, and imitation learning of action behaviors [,]. In addition, the functional areas of language function and mirror neuron system overlapped in the left Inferior Frontal Gyrus (IFG), ventral premotor cortex, parietal lobule cortex, and superior temporal sulcus cortex []. According to the theory of speech action perception, the production of a sentence or a single time involving an action will produce a mapping in the cerebral motor cortex under the corresponding word meaning, which may promote the recovery of hand motor function, and the MNS system may play a mediating role in the interactive promotion process of the two []. This offers a new strategic approach for the research and application of MNS theory in the rehabilitation of motor and language functions after stroke.
The recovery of aphasia is a dynamic and nonlinear process. Language function can recover spontaneously to a certain extent within 3 months after stroke, but most patients leave varying degrees of chronic language dysfunction. The progress of early language function recovery depends on blood flow reperfusion, and the degree of edema and local inflammation around the damaged brain area further affects functional recovery []. In stroke patients in the subacute and chronic phases, the recovery effect of language ability is closely related to the remodeling of language functional areas and the compensation of other functional areas []. Hand motor dysfunction after stroke can be improved to varying degrees after treatment. With the progress of time after a stroke, spontaneous repair gradually reaches a plateau. However, chronic hand motor function can still be improved by rehabilitation training. Similarly, the recovery mechanism is also based on neuroplasticity. It can be seen from the above that the neural rehabilitation mechanism of hand motor function is extremely similar to the process and mechanism of aphasia language function recovery. Based on the existence of functional connectivity and interaction between language and hand movement brain representation areas, some researchers have studied the efficacy and mechanism of comprehensive rehabilitation treatment for patients with post-stroke aphasia and hand movement dysfunction. The intervention methods used are different, and the research conclusions are not completely consistent, but preliminary research results have been obtained.
Treatment methods based on MNS theory are widely used in the motor function rehabilitation of stroke. To verify whether the MNS system can be activated to promote the recovery of language function in aphasia patients, most studies observe hand movements and repeat the movements at the same time. Research results show that hand movement observation training, no matter in video [,] or real scene [], has a certain promotion effect on the language function of patients with transcortical motor aphasia, and the improvement effect is more significant than that of static pictures. Its promotion is mainly manifested in the extraction and expression of words []. Beilock, et al. [] believed that its promoting effect on language comprehension was through the activation of the left premotor cortex. Yang et al. adopted a more complex movement training, which required subjects to perform wrist and finger movement training while observing their hand movements. The research results showed that hand movement training effectively helped aphasia patients recover their overall language function, but the rehabilitation effects of different types of aphasia patients were different.
The left and right hemispheres of the human brain have their advantages, and most people show left hemisphere dominance. Meanwhile, traditional language theory holds that “we speak with the left side of the brain”, and Broca’s area is mainly involved in speech production. Most studies have shown that the non-dominant hemisphere interferes with the dominant hemisphere’s language rehabilitation mechanism and thus hinders the recovery of aphasia []. Repetitive transcranial Magnetic Stimulation (rTMS) [,] found that compared with controls, Low-frequency rTMS can significantly improve the accuracy of picture naming and description in aphasia patients by inhibiting the excitability of the right inferior frontal gyrus triangle, and the changes of brain excitability and synaptic connectivity can last for a long time. Further findings [,] suggest that in subacute post-stroke aphasia, the homology of Broca’s area in the non-dominant hemisphere may affect the neuroplasticity of the language network to a certain extent. The results of this series of studies also provide support for the above theory. However, when the dominant hemisphere is severely damaged, the transfer of language function to the non-dominant hemisphere is one of the possible mechanisms for its rehabilitation [,], and the right cerebral hemisphere is involved in the recovery of specific functions of speech []. At the same time, the compensatory effect was further enhanced by applying excitatory stimulation to the right hemisphere. Janabi, et al. [] used a combination of excitatory rTMS and melodic tone therapy in two patients with non-fluent aphasia, in which one participant showed an improvement in oral fluency. However, Heikkinen [] combined ILAT (Intensive language-action Therapy) and rTMS or sham-rTMS to conduct speech function rehabilitation training for patients, and compared the improvement of speech and Language function between the experimental group and the control group, the difference was not statistically significant. In addition, Xu, et al. [] used theta pulse to stimulate the left M1 hand motor representative area of patients with aphasia and hand movement disorders after stroke. fMRI was used to observe the central excitability changes before and after stimulation, and it was found that excitatory stimulation of this node could induce excitatory changes in the cortical language area. This suggests that this location may be a potential stimulation target for the speech-motor synergistic recovery phenotype in stroke patients. However, the different stages of functional recovery after stroke, the length of the disease course of the patients included in the study, the effect of handedness, and the sample size all affect the results of the study. The role of the right cerebral hemisphere in the rehabilitation of language function still needs to be further explored.
Transcranial Direct Current Stimulation (tDCS) uses a weak current to regulate the activity of neural cells in the cerebral cortex. Through anodal stimulation, the excitability of the M1 area in the affected hemisphere is increased, or cathodal stimulation, the excitability of the M1 area in the contralateral hemisphere is inhibited. The motor cortex activity of the two hemispheres is balanced so that the complex function of the hand can be restored to a certain extent. In addition, anodal tDCS stimulation of the left frontal lobe or left hemisphere Wernicke area combined with intensive language training can significantly improve the naming accuracy and fluency of patients [,]. As an intervention method, tDCS has been widely studied in the recovery of hemiplegic hand motor dysfunction and aphasia respectively, but the research on the intervention mechanism and therapeutic effect of the combination of the above two dysfunctions is still in the exploratory stage. Based on the interaction between language and the motor system, M1-tDCS stimulates the motor system that is functionally connected to the language network. It does not require pre-processing imaging to identify individual language areas and is easier to operate and manage than traditional tDCS. To explore its curative effect, some researchers have used M1-tDCS to intervene in patients with post-stroke aphasia and explored its possible mechanism. The results show that M1-tDCS can improve the activity and language communication function of patients with aphasia to varying degrees, and can improve the retrieval function of action-related words, which can last for a long time [,]. The observed positive effects could be the result of direct effects of tDCS on the motor cortex or other regions underlying the positive electrode, or M1-tDCS could affect premotor and frontal regions that are related to the function of the speech production task. This conjecture was also supported by the results that M1-tDCS performed fMRI while performing the word retrieval task, and the task-related activities were regulated by the bilateral prefrontal language areas []. Therefore, the mechanism by which M1-tDCS promotes the recovery of speech function may be to promote the functional processing of the language areas that are preserved after the lesion.
The discovery of the MNS offers a theoretical foundation and novel insights for the rehabilitation of post-stroke residual dysfunction. It has been widely recognized that there is a behavioral and neuroscientific connection between language and hand-motor function. Therefore, the combined language-hand movement synchronous training for patients with aphasia may have great potential. In the case of hand motor training, it is necessary to understand more about the observation of executive movement or the effect of hand movement itself on language function. In addition, based on the complex relationship between hand motor function and language network, more longitudinal neuroimaging studies are needed to explore the functional recovery of aphasia after stroke, and non-invasive brain stimulation technology is needed to explore and develop stimulation targets and treatment plans for patients with combined language and hand motor dysfunction.
Grönberg A, Henriksson I, Stenman M. Incidence of Aphasia in Ischemic Stroke. Neuroepidemiology. 2022; 56(3): 174-182.
Lawrence ES, Coshall C, Dundas R. Estimates of the prevalence of acute stroke impairments and disability in a multiethnic population. Stroke. 2001; 32(6):1279-1284.
Zhou X, Qu L, Zhang W. Analysis of Proteomic Characteristics of Peripheral Blood in Preeclampsia and Study of Changes in Fetal Arterial Doppler Parameters Based on Magnetic Nanoparticles. Computational and mathematical methods in medicine. 2021; 2021: 7145487.
Edelkraut L, López-Barroso D, Torres-Prioris MJ. Spectrum of neuropsychiatric symptoms in chronic post-stroke aphasia. World journal of psychiatry. 2022; 12(3): 450-469.
Anderlini D, Wallis G, Marinovic W. Language as a Predictor of Motor Recovery: The Case for a More Global Approach to Stroke Rehabilitation. Neurorehabilitation and neural repair. 2019; 33(3): 167-178.
Wortman-Jutt S, Edwards D. Poststroke Aphasia Rehabilitation: Why All Talk and No Action? Neurorehabilitation and neural repair. 2019.
Xu S, Yan Z, Pan Y. Associations between Upper Extremity Motor Function and Aphasia after Stroke: A Multicenter Cross-Sectional Study. Behavioural Neurology. 2021; 2021: 1-10.
Thibault S, Py R, Gervasi AM. Tool use and language share syntactic processes and neural patterns in the basal ganglia. Science. 2021; 374(6569).
Gough PM, Riggio L, Chersi F. Nouns referring to tools and natural objects differentially modulate the motor system. Neuropsychologia. 2012; 50(1): 19-25.
Cobble M. Language impairment in motor neurone disease. Journal of the neurological sciences. 1998;160 Suppl 1: S47-S52.
Bak TH, Hodges JR. The effects of motor neurone disease on language: further evidence. Brain and language. 2004; 89(2): 354-361.
Wan CY, Demaine K, Zipse L. From music making to speaking: engaging the mirror neuron system in autism. Brain research bulletin. 2010; 82(3-4): 161-168.
Miyahara M, Kitada R, Sasaki AT. From gestures to words: spontaneous verbal labeling of complex sequential hand movements reduces fMRI activation of the imitation-related regions. Neuroscience research. 2013; 75(3): 228-238.
Rizzolatti G, Fadiga L, Matelli M. Localization of grasp representations in humans by PET: 1. Observation versus execution. Experimental brain research. 1996;111(2): 246-252.
Rizzolatti G, Luppino G, Matelli M. The organization of the cortical motor system: new concepts. Electroencephalography and clinical neurophysiology. 1998; 106(4): 283-296.
Rizzolatti G, Arbib MA. Language within our grasp. Trends in neurosciences, 1998; 21(5): 188-194.
Jo S, Kim H, Song C. A Novel Approach to Increase Attention during Mirror Therapy among Stroke Patients: A Video-Based Behavioral Analysis. Brain sciences. 2022;12(3).
Dai C, Peng Z, Wang L. Total sleep deprivation reduces the table tennis anticipation performance of young men: A functional magnetic resonance imaging study. iScience. 2023; 26(10): 107973.
Von Heiseler TN. Syntax of Testimony: Indexical Objects, Syntax, and Language or How to Tell a Story Without Words. Frontiers in psychology. 2019;10: 477.
Hesling I, Labache L, Joliot M. Large-scale plurimodal networks common to listening to, producing and reading word lists: an fMRI study combining task-induced activation and intrinsic connectivity in 144 right-handers. Brain structure & function. 2019; 224(9): 3075-3094.
Ochfeld E, Newhart M, Molitoris J. Ischemia in broca area is associated with broca aphasia more reliably in acute than in chronic stroke. Stroke. 2010; 41(2): 325-330.
Kiran S. What is the nature of poststroke language recovery and reorganization? ISRN neurology. 2012; 2012: 786872.
Chen W, Ye Q, Zhang S. Aphasia rehabilitation based on mirror neuron theory: a randomized-block-design. Neural regeneration research. 2019;14(6): 1004-1012.
Chen W, Ye Q, Ji X. Mirror neuron system based therapy for aphasia rehabilitation. Frontiers in psychology. 2015; 6: 1665.
Gili T, Fiori V, De Pasquale G. Right sensory-motor functional networks subserve action observation therapy in aphasia. Brain imaging and behavior. 2017;11(5): 1397-1411.
Marangolo P, Bonifazi S, Tomaiuolo F. Improving language without words: first evidence from aphasia. Neuropsychologia. 2010; 48(13): 3824-3833.
Beilock SL, Lyons IM, Mattarella-Micke A. Sports experience changes the neural processing of action language. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(36): 13269-13273.
Turkeltaub PE. Brain Stimulation and the Role of the Right Hemisphere in Aphasia Recovery. Current neurology and neuroscience reports. 2015; 15(11): 72.
Barwood CHS, Murdoch BE, Riek S. Long term language recovery subsequent to low frequency rTMS in chronic non-fluent aphasia. NeuroRehabilitation. 2013; 32(4): 915-928.
Thiel A, Hartmann A, Rubi-Fessen I. Effects of noninvasive brain stimulation on language networks and recovery in early poststroke aphasia. Stroke. 2013; 44(8): 2240-2246.
Naeser MA, Martin PI, Nicholas M. Improved picture naming in chronic aphasia after TMS to part of right Broca's area: an open-protocol study. Brain and language. 2005; 93(1): 95-105.
Hartwigsen G, Saur D, Price CJ. Perturbation of the left inferior frontal gyrus triggers adaptive plasticity in the right homologous area during speech production. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(41): 16402-16407.
Pulvermüller F, Schönle P W. Behavioral and neuronal changes during treatment of mixed transcortical aphasia: a case study. Cognition. 1993; 48(2): 139-161.
Saur D, Hartwigsen G. Neurobiology of language recovery after stroke: lessons from neuroimaging studies. Archives of physical medicine and rehabilitation. 2012; 93(1 Suppl): S15-S25.
Vargha-Khadem F, Carr LJ, Isaacs E. Onset of speech after left hemispherectomy in a nine-year-old boy. Brain: a journal of neurology. 1997;120 (Pt 1): 159-182.
Al-Janabi S, Nickels LA, Sowman PF. Augmenting melodic intonation therapy with non-invasive brain stimulation to treat impaired left-hemisphere function: two case studies. Frontiers in psychology. 2014; 5: 37.
Heikkinen PH, Pulvermüller F, Mäkelä JP. Combining rTMS with Intensive Language-Action Therapy in Chronic Aphasia: A Randomized Controlled Trial. 2018; 12:1036.
Xu S, Yang Q, Chen M. Capturing Neuroplastic Changes after iTBS in Patients with Post-Stroke Aphasia: A. Brain sciences. 2021;11(11).
Baker JM, Rorden C, Fridriksson J. Using transcranial direct-current stimulation to treat stroke patients with aphasia. Stroke. 2010; 41(6): 1229-1236.
Fiori V, Coccia M, Marinelli CV. Transcranial direct current stimulation improves word retrieval in healthy and nonfluent aphasic subjects. Journal of cognitive neuroscience. 2011; 23(9): 2309-2323.
Meinzer M, Darkow R, Lindenberg R. Electrical stimulation of the motor cortex enhances treatment outcome in post-stroke aphasia. Brain: a journal of neurology. 2016;139(Pt 4): 1152-1163.
Meinzer M, Lindenberg R, Sieg MM. Transcranial direct current stimulation of the primary motor cortex improves word-retrieval in older adults. Frontiers in aging neuroscience. 2014; 6: 253.
Xu S, Liang C, Chen S, Huang Z, Jiang H. Association and New Therapy Perspectives in Post-Stroke Aphasia with Hand Motor Dysfunction. IgMin Res. Jan 25, 2024; 2(1): 032-035. IgMin ID: igmin141; DOI: 10.61927/igmin141; Available at: www.igminresearch.com/articles/pdf/igmin141.pdf
次のリンクを共有した人は、このコンテンツを読むことができます:
Address Correspondence:
Haoqing Jiang, MS, Department of Rehabilitation Medicine, Zhangzhou Municipal Hospital of Fujian Medical University, 59 West Shengli Road, Xiangcheng District, 363000, Zhangzhou, Fujian, China. Email: [email protected]
How to cite this article:
Xu S, Liang C, Chen S, Huang Z, Jiang H. Association and New Therapy Perspectives in Post-Stroke Aphasia with Hand Motor Dysfunction. IgMin Res. Jan 25, 2024; 2(1): 032-035. IgMin ID: igmin141; DOI: 10.61927/igmin141; Available at: www.igminresearch.com/articles/pdf/igmin141.pdf
Copyright: © 2024 Xu S, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
 Figure 1: Number of literature searches for keywords related...
Figure 1: Number of literature searches for keywords related...
Grönberg A, Henriksson I, Stenman M. Incidence of Aphasia in Ischemic Stroke. Neuroepidemiology. 2022; 56(3): 174-182.
Lawrence ES, Coshall C, Dundas R. Estimates of the prevalence of acute stroke impairments and disability in a multiethnic population. Stroke. 2001; 32(6):1279-1284.
Zhou X, Qu L, Zhang W. Analysis of Proteomic Characteristics of Peripheral Blood in Preeclampsia and Study of Changes in Fetal Arterial Doppler Parameters Based on Magnetic Nanoparticles. Computational and mathematical methods in medicine. 2021; 2021: 7145487.
Edelkraut L, López-Barroso D, Torres-Prioris MJ. Spectrum of neuropsychiatric symptoms in chronic post-stroke aphasia. World journal of psychiatry. 2022; 12(3): 450-469.
Anderlini D, Wallis G, Marinovic W. Language as a Predictor of Motor Recovery: The Case for a More Global Approach to Stroke Rehabilitation. Neurorehabilitation and neural repair. 2019; 33(3): 167-178.
Wortman-Jutt S, Edwards D. Poststroke Aphasia Rehabilitation: Why All Talk and No Action? Neurorehabilitation and neural repair. 2019.
Xu S, Yan Z, Pan Y. Associations between Upper Extremity Motor Function and Aphasia after Stroke: A Multicenter Cross-Sectional Study. Behavioural Neurology. 2021; 2021: 1-10.
Thibault S, Py R, Gervasi AM. Tool use and language share syntactic processes and neural patterns in the basal ganglia. Science. 2021; 374(6569).
Gough PM, Riggio L, Chersi F. Nouns referring to tools and natural objects differentially modulate the motor system. Neuropsychologia. 2012; 50(1): 19-25.
Cobble M. Language impairment in motor neurone disease. Journal of the neurological sciences. 1998;160 Suppl 1: S47-S52.
Bak TH, Hodges JR. The effects of motor neurone disease on language: further evidence. Brain and language. 2004; 89(2): 354-361.
Wan CY, Demaine K, Zipse L. From music making to speaking: engaging the mirror neuron system in autism. Brain research bulletin. 2010; 82(3-4): 161-168.
Miyahara M, Kitada R, Sasaki AT. From gestures to words: spontaneous verbal labeling of complex sequential hand movements reduces fMRI activation of the imitation-related regions. Neuroscience research. 2013; 75(3): 228-238.
Rizzolatti G, Fadiga L, Matelli M. Localization of grasp representations in humans by PET: 1. Observation versus execution. Experimental brain research. 1996;111(2): 246-252.
Rizzolatti G, Luppino G, Matelli M. The organization of the cortical motor system: new concepts. Electroencephalography and clinical neurophysiology. 1998; 106(4): 283-296.
Rizzolatti G, Arbib MA. Language within our grasp. Trends in neurosciences, 1998; 21(5): 188-194.
Jo S, Kim H, Song C. A Novel Approach to Increase Attention during Mirror Therapy among Stroke Patients: A Video-Based Behavioral Analysis. Brain sciences. 2022;12(3).
Dai C, Peng Z, Wang L. Total sleep deprivation reduces the table tennis anticipation performance of young men: A functional magnetic resonance imaging study. iScience. 2023; 26(10): 107973.
Von Heiseler TN. Syntax of Testimony: Indexical Objects, Syntax, and Language or How to Tell a Story Without Words. Frontiers in psychology. 2019;10: 477.
Hesling I, Labache L, Joliot M. Large-scale plurimodal networks common to listening to, producing and reading word lists: an fMRI study combining task-induced activation and intrinsic connectivity in 144 right-handers. Brain structure & function. 2019; 224(9): 3075-3094.
Ochfeld E, Newhart M, Molitoris J. Ischemia in broca area is associated with broca aphasia more reliably in acute than in chronic stroke. Stroke. 2010; 41(2): 325-330.
Kiran S. What is the nature of poststroke language recovery and reorganization? ISRN neurology. 2012; 2012: 786872.
Chen W, Ye Q, Zhang S. Aphasia rehabilitation based on mirror neuron theory: a randomized-block-design. Neural regeneration research. 2019;14(6): 1004-1012.
Chen W, Ye Q, Ji X. Mirror neuron system based therapy for aphasia rehabilitation. Frontiers in psychology. 2015; 6: 1665.
Gili T, Fiori V, De Pasquale G. Right sensory-motor functional networks subserve action observation therapy in aphasia. Brain imaging and behavior. 2017;11(5): 1397-1411.
Marangolo P, Bonifazi S, Tomaiuolo F. Improving language without words: first evidence from aphasia. Neuropsychologia. 2010; 48(13): 3824-3833.
Beilock SL, Lyons IM, Mattarella-Micke A. Sports experience changes the neural processing of action language. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(36): 13269-13273.
Turkeltaub PE. Brain Stimulation and the Role of the Right Hemisphere in Aphasia Recovery. Current neurology and neuroscience reports. 2015; 15(11): 72.
Barwood CHS, Murdoch BE, Riek S. Long term language recovery subsequent to low frequency rTMS in chronic non-fluent aphasia. NeuroRehabilitation. 2013; 32(4): 915-928.
Thiel A, Hartmann A, Rubi-Fessen I. Effects of noninvasive brain stimulation on language networks and recovery in early poststroke aphasia. Stroke. 2013; 44(8): 2240-2246.
Naeser MA, Martin PI, Nicholas M. Improved picture naming in chronic aphasia after TMS to part of right Broca's area: an open-protocol study. Brain and language. 2005; 93(1): 95-105.
Hartwigsen G, Saur D, Price CJ. Perturbation of the left inferior frontal gyrus triggers adaptive plasticity in the right homologous area during speech production. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(41): 16402-16407.
Pulvermüller F, Schönle P W. Behavioral and neuronal changes during treatment of mixed transcortical aphasia: a case study. Cognition. 1993; 48(2): 139-161.
Saur D, Hartwigsen G. Neurobiology of language recovery after stroke: lessons from neuroimaging studies. Archives of physical medicine and rehabilitation. 2012; 93(1 Suppl): S15-S25.
Vargha-Khadem F, Carr LJ, Isaacs E. Onset of speech after left hemispherectomy in a nine-year-old boy. Brain: a journal of neurology. 1997;120 (Pt 1): 159-182.
Al-Janabi S, Nickels LA, Sowman PF. Augmenting melodic intonation therapy with non-invasive brain stimulation to treat impaired left-hemisphere function: two case studies. Frontiers in psychology. 2014; 5: 37.
Heikkinen PH, Pulvermüller F, Mäkelä JP. Combining rTMS with Intensive Language-Action Therapy in Chronic Aphasia: A Randomized Controlled Trial. 2018; 12:1036.
Xu S, Yang Q, Chen M. Capturing Neuroplastic Changes after iTBS in Patients with Post-Stroke Aphasia: A. Brain sciences. 2021;11(11).
Baker JM, Rorden C, Fridriksson J. Using transcranial direct-current stimulation to treat stroke patients with aphasia. Stroke. 2010; 41(6): 1229-1236.
Fiori V, Coccia M, Marinelli CV. Transcranial direct current stimulation improves word retrieval in healthy and nonfluent aphasic subjects. Journal of cognitive neuroscience. 2011; 23(9): 2309-2323.
Meinzer M, Darkow R, Lindenberg R. Electrical stimulation of the motor cortex enhances treatment outcome in post-stroke aphasia. Brain: a journal of neurology. 2016;139(Pt 4): 1152-1163.
Meinzer M, Lindenberg R, Sieg MM. Transcranial direct current stimulation of the primary motor cortex improves word-retrieval in older adults. Frontiers in aging neuroscience. 2014; 6: 253.