
Kinetic Study of the Removal of Reafix Yellow B8G Dye by Boiler Ash
Industrial Engineering Materials Science受け取った 30 Nov 2023 受け入れられた 12 Dec 2023 オンラインで公開された 13 Dec 2023
Focusing on Biology, Medicine and Engineering ISSN: 2995-8067 | Quick Google Scholar
Next Full Text
Influence of Polycarboxylate Superplasticizer on the Calorimetric and Physicomechanical Properties of Mortar
Previous Full Text
Deep Semantic Segmentation New Model of Natural and Medical Images


受け取った 30 Nov 2023 受け入れられた 12 Dec 2023 オンラインで公開された 13 Dec 2023
The textile sector has great relevance in the Brazilian socioeconomic context, generating large volumes of effluents of varied composition, with dyes being one of the most worrying contaminants due to their characteristics of high solubility, harmfulness and low degradability. Among them the Reafix Yellow B8G (ARB8G) dye has an azo nature, harmful to organisms in the aquatic environment. According to Brazilian laws, it is recommended to dispose of waste through incineration or landfill, due to its resistance to biodegradation. One of the methods for treating effluents is the adsorption of contaminants using materials with adsorption capacity, such as residue from the burning of biomass in boilers (ash). The general objective of this work was to evaluate the adsorption kinetics by contact time of the ARB8G dye by boiler ash. The test was based on mixing the adsorbent material with a dye solution at 75 mg.L-1, at constant temperature and stirring at different time intervals. To quantify the amount of adsorbed dye, the UV-Vis spectrometry method was used. The kinetic test indicated an equilibrium time of 30 minutes, with 100% removal under the tested conditions. From this result and considering that boiler residue is an underutilized and low-cost material, it is concluded that this substance has significant potential to be applied as an alternative material for the treatment of effluents containing dyes.
Since the industrial revolution, technologies and innovations in industrial processes have experienced significant growth, providing the development of quality products on a large scale, in addition to generating jobs and growing the world economy. However, combined with this, concern arose about how the waste generated in the process would be treated, leading to a discussion about environmental impacts and how they affected human health. Therefore, the United Nations (UN) included in the Sustainable Development Goals (SDGs) and in the 2030 Agenda goals to guarantee sustainable consumption and production patterns through the Circular Economy with prevention, reduction, recycling and reuse actions for the waste [].
In this context, the waste studied in this work is the ash generated by the burning of biomass in industrial boilers and, generally, its main destination is industrial landfills. However, some types of ash may contain high levels of unburned carbonaceous material with high porosity, providing the residue with adsorbent characteristics and being able to remove contaminants of a toxic nature [] such as Reafix B8G Yellow dye.
According to the Chemical Product Safety Information Sheet [], the Reafix B8G Yellow dye is azo in nature, harmful to organisms in the aquatic environment, and may cause long-term adverse effects. Therefore, it is recommended to dispose of waste through incineration or landfill, due to its resistance to biodegradation.
When effluent contaminated with dyes is discarded without treatment into water bodies, they alter the physical-chemical properties of the environment. The dyes present impair light penetration, compromising photosynthesis and consequently the amount of dissolved oxygen [].
Thus, considering the need for new applications for waste originating from industrial processes combined with the concepts of Circular Economy and the SDGs, this study proposed the evaluation of ash produced by the burning of biomass as an adsorbent material for the Reafix B8G Yellow dye.
Adsorption was evaluated by the amount of yellow dye adsorbed per contact time with the residue. The analyzes were carried out in triplicate at each time point and consisted of adding 0.15 g of activated carbon to 25 mL of dye solution with a concentration of 75 mg.L-1. The dye used was provided by the company AGS Química and the aqueous solutions were prepared by dissolving the solid dye in distilled water.
The test was carried out in a thermostatic shaker, under agitation of 130 rpm and a temperature of 30 ºC at constant pressure. After the contact time, the samples were centrifuged at 3000 rpm for 10 min, then they were subjected to Visible Ultraviolet (UV-Vis) spectrometry at wavelength 425 nm, based on the study by Silva [] who used the same dye in her study. Using the absorbance values, the concentration of dye remaining in the sample was calculated and consequently the amount of dye adsorbed per gram of activated carbon.
The results obtained in the kinetic test conducted at the natural pH of the solution (5.84) are represented in Figure 1.
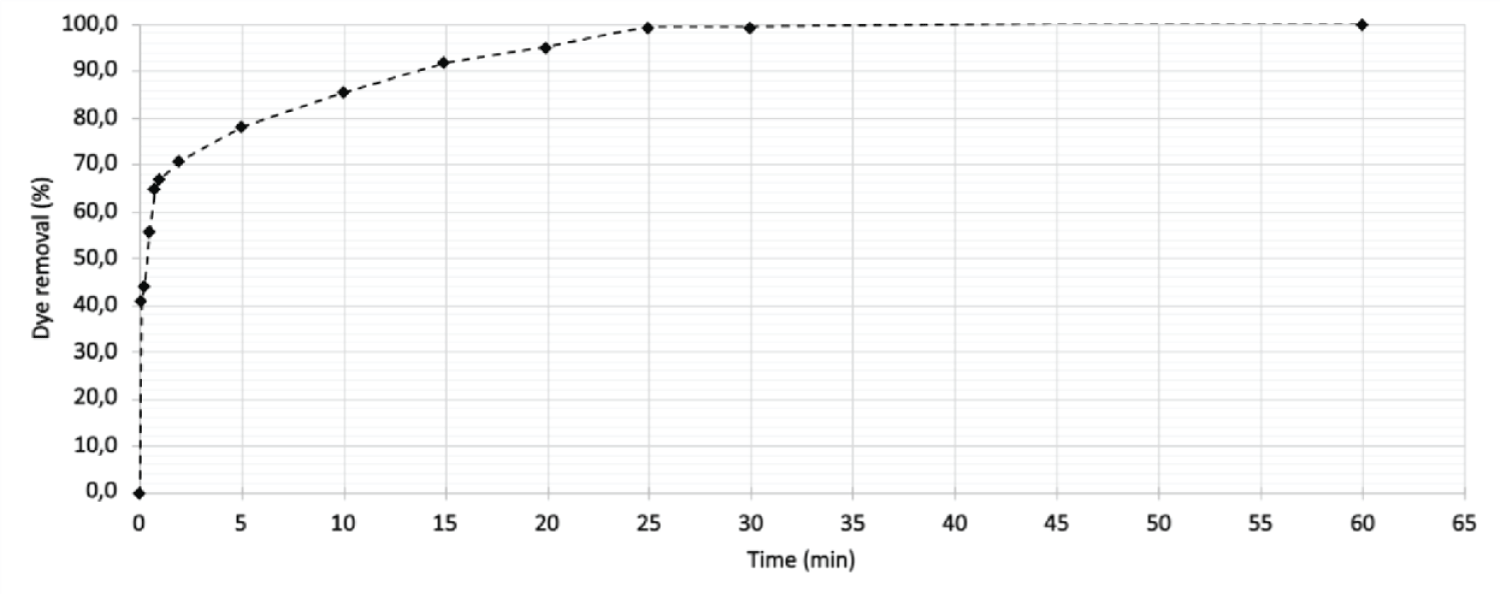 Figure 1: Adsorption kinetics of Reafix B8G yellow dye solution (75 mg.L-1) in the presence of boiler ash (0.15 g). The data were obtained keeping the experimental conditions constant, varying only the contact time between the phases.
Figure 1: Adsorption kinetics of Reafix B8G yellow dye solution (75 mg.L-1) in the presence of boiler ash (0.15 g). The data were obtained keeping the experimental conditions constant, varying only the contact time between the phases.Considering the information contained in the graphs, dye removal is more accelerated in the first moments, obtaining 90% removal in the first 15 minutes and 100% after 30 minutes.
From the experimental results, it is noted that adsorption is faster at the beginning, followed by slow sorption until reaching the equilibrium period. This behavior is observed in studies such as Hawerroth [], in which approximately 70% of the yellow dye is removed in the first 10 minutes using a mineral byproduct from the detonation of granite rocks as an adsorbent.
Silva [] in his study found similar results for dye removal with malt pomace using the same contact time method but varying the temperature. The method proved to be efficient and found 30 degrees Celsius as the optimal adsorption temperature where removal was 72.5% in 15 minutes.
This can be explained by the relationship between the number of empty sites on the surface of the adsorbent material and time. At the beginning of the adsorption process there is a greater number of empty sites that are available to be filled, and over time this ratio decreases, generating repulsion and making the adsorption process difficult [].
According to Raganati, et al. [] fast kinetics is important from an industrial point of view as it enables the use of smaller volume reactors, promoting reduced operational costs and high efficiency.
The kinetic test showed that the ash analyzed reaches equilibrium in 30 minutes, removing 100% of the dye, proving to be very efficient for this purpose. Considering that boiler residue is an underused and low-cost material, it is concluded that this substance has significant potential to be applied directly to the adsorption of dyes in effluents.
For best results it is necessary to test the influence of pH and temperature, as these conditions directly influence the adsorption process. Furthermore, it is interesting to carry out the kinetic evaluation of adsorption using real industrial effluents that contain dye in their composition.
The results show that the residue from burning biomass to generate energy can be reused in the treatment of effluents with dye. This directly aligns with the concept of a circular economy that uses waste as an input in a new production process.
DESA U. The Sustainable Development Goals Report 2023: Special Edition-July 2023. New York, USA. 2023.
Aigbe UO, Ukhurebor KE, Onyancha RB, Osibote OA, Darmokoesoemo H, Kusuma HS. Fly ash-based adsorbent for adsorption of heavy metals and dyes from aqueous solution: a review. Journal of Materials Research and Technology. 2021; 14:2751-2774.
FISPQ nº 0222/08 of 2008. Chemical Product Safety Data Sheet: Reafix Yellow. Barueri, SP: AGS Química.
Martins TMDA. Adsorption of dyes and metals and the use of waste using biosorbents: a review. 2020.
Silva BCD. Biosorption of Reafix B8G Yellow dye from malt bagasse in batch and continuous system: experimental evaluation and computational fluid dynamic simulation (Master's thesis, Federal Technological University of Paraná). 2019.
Hawerroth M. Granite rock powder in the removal of dye in effluent and application in Portland cement matrix (Master's thesis, Federal Technological University of Paraná). 2023.
Santos J, Silva M. Adsorption of Methylene Blue Using Rice Husk. In Proceedings of the XIII COBEQ–IC 2019-Brazilian Congress of Chemical Engineering in Scientific Initiation. 2019; 19461952.
Raganati F, Miccio F, Ammendola P. Adsorption of carbon dioxide for post-combustion capture: A review. energy & fuels. 2021; 35(16):12845-12868.
Prinz PE, Hawerroth M, Lima LSD, Kinetic Study of the Removal of Reafix Yellow B8G Dye by Boiler Ash. IgMin Res. 13 Dec, 2023; 1(2): 130-132. IgMin ID: igmin127; DOI: 10.61927/igmin127; Available at: www.igminresearch.com/articles/pdf/igmin127.pdf
次のリンクを共有した人は、このコンテンツを読むことができます:
1Departament of Chemical Engineering, Federal University of Technology-Paraná, Ponta Grossa, Paraná, Brazil
2Alphacarbo Industrial Ltda, Guarapuava, Ponta Grossa, Paraná, Brazil
Address Correspondence:
Juliana Martins Teixeira de Abreu Pietrobelli, Departament of Chemical Engineering, Federal University of Technology-Paraná, Ponta Grossa, Paraná, Brazil, Email: [email protected]
How to cite this article:
Prinz PE, Hawerroth M, Lima LSD, Kinetic Study of the Removal of Reafix Yellow B8G Dye by Boiler Ash. IgMin Res. 13 Dec, 2023; 1(2): 130-132. IgMin ID: igmin127; DOI: 10.61927/igmin127; Available at: www.igminresearch.com/articles/pdf/igmin127.pdf
Copyright: © 2023 Prinz PF, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
 Figure 1: Adsorption kinetics of Reafix B8G yellow dye solut...
Figure 1: Adsorption kinetics of Reafix B8G yellow dye solut...
DESA U. The Sustainable Development Goals Report 2023: Special Edition-July 2023. New York, USA. 2023.
Aigbe UO, Ukhurebor KE, Onyancha RB, Osibote OA, Darmokoesoemo H, Kusuma HS. Fly ash-based adsorbent for adsorption of heavy metals and dyes from aqueous solution: a review. Journal of Materials Research and Technology. 2021; 14:2751-2774.
FISPQ nº 0222/08 of 2008. Chemical Product Safety Data Sheet: Reafix Yellow. Barueri, SP: AGS Química.
Martins TMDA. Adsorption of dyes and metals and the use of waste using biosorbents: a review. 2020.
Silva BCD. Biosorption of Reafix B8G Yellow dye from malt bagasse in batch and continuous system: experimental evaluation and computational fluid dynamic simulation (Master's thesis, Federal Technological University of Paraná). 2019.
Hawerroth M. Granite rock powder in the removal of dye in effluent and application in Portland cement matrix (Master's thesis, Federal Technological University of Paraná). 2023.
Santos J, Silva M. Adsorption of Methylene Blue Using Rice Husk. In Proceedings of the XIII COBEQ–IC 2019-Brazilian Congress of Chemical Engineering in Scientific Initiation. 2019; 19461952.
Raganati F, Miccio F, Ammendola P. Adsorption of carbon dioxide for post-combustion capture: A review. energy & fuels. 2021; 35(16):12845-12868.