
Benzo[4’,5’]imidazo[2’,1’:6,1]pyrido[2,3-d]pyrimidines: Past and Present
Biochemistry受け取った 01 Nov 2023 受け入れられた 13 Nov 2023 オンラインで公開された 16 Nov 2023
Focusing on Biology, Medicine and Engineering ISSN: 2995-8067 | Quick Google Scholar
Previous Full Text
Federated Learning- Hope and Scope


受け取った 01 Nov 2023 受け入れられた 13 Nov 2023 オンラインで公開された 16 Nov 2023
Synthetic approaches to the construction of the benzo[4’,5’]imidazo[2’,1’:6,1]pyrido[2,3-d]pyrimidine system based on heterocyclizations of substituted benzimidazoles and a new alternative strategy based on 2,4,6-trisubstituted pyrimidinyl-5-propanoic acids are considered. The latter method has been shown to be a successful addition to previously described methods, since it allows one to bypass the significant limitations associated with the use of substituted benzimidazoles and allows the introduction of functional substituents at different positions of the heterocycle that are inaccessible by other methods. The available information on derivatives of this heterocyclic system and their biological properties is summarized.
The development of synthetic methods for the construction of polycyclic azaheterocycles is the focus of modern research in the chemistry of heterocyclic compounds. The role of heterocyclic compounds, in particular nitrogen-containing heterocycles, in modern medical and pharmacological chemistry as a reliable structural basis for the search for new effective drugs is especially noticeable [-]. It is known that currently more than 90% of new drugs are heterocycles, and the study of the mechanism of action of drugs, in turn, makes it possible to elucidate the molecular mechanisms of specific biochemical processes, making the connection between the organic chemistry of heterocycles, bioorganic chemistry and biochemistry reciprocal.
It is worth noting the importance of the most common azaheterocycles in nature, such as pyrimidines and condensed pyrimidines (purines, pteridines), pyridines, benzimidazoles, which are essential components of all living organisms as nucleic acid bases, coenzymes, mediators of intracellular signals, storage devices and carriers of high-energy phosphates, etc. It is not surprising that synthetic nitrogen-containing heterocycles, which are structural analogues of biologically active natural compounds, are considered as privileged structures in the synthesis of physiologically Active compounds.
Moreover, annulation of various heterocycles leads to polycondensed compounds with a planar structure and a unique electronic circuit, which combine the structural motifs of various pharmacophores in one molecule, which allows us to expect new interesting physicochemical and biological properties from polycyclic compounds that are not characteristic of the original heterocyclic systems.
It is well known, that polycyclic heteroaromatic compounds based on annelated azaheterocycles, the most important structural feature of which is the planar structure, exhibit high biological activity, including antitumor, antibacterial, antiviral and others []. The biological activity of this class of compounds is due to their ability to interact with DNA, being associated with small and large grooves or intercalation between adjacent bases in a double helix, the interaction mechanism of the latter being considered as the main one. In both cases, the secondary structure of DNA is distorted and its functioning is disrupted, and therefore the connections with this mechanism of action are considered as the most promising in developing new-generation drugs for the treatment of tumor diseases and viral and bacterial infections. It should be noted that bi-and tricyclic compounds are best known as intercalating heterocycles, while tetra- and higher-annealed compounds are less well studied, although the possibility of intercalation and the associated pharmacological activity are shown for them [].
In contrast to the relatively well-studied bi- and tricyclic azaheterocycles, tetra- and more highly annealed azaheterocycles have been studied much less well, although the possibility of interaction with nucleic acid bases via the intercalation mechanism has been shown for them [-].
Among the tetracyclic heteroaromatic compounds, we have drawn attention to the syntheses and biological properties of the derivatives of the benzo[4′,5′] imidazo[2’,1’:6,1]pyrido[2,3-d]pyrimidine (1) condensed on the basis of three nitrogen-containing heterocycles, and of which there are a limited number of publications in the literature.
Before moving on to a discussion of work in this area, it is appropriate to briefly dwell on the names of the heterocyclic system 1 available in the chemical literature, since here we encounter an example of an alternative name for a chemical compound according to the nomenclature rules of the Chemical Abstract Service (CAS) and IUPAC. In the Index of Ring Systems CAS, this heterocyclic system was first registered as pyrimido[5’,4’: 5,6]pyrido[1,2-a] benzimidazole [], while in later reports the same system is found under the name benzo[4,5]imidazo[2’,1’:6,1] pyrido[2,3-d]pyrimidin, generated in the nomenclature program ACD/Name , package ACD / Chem Sketch 1.0, according to IUPAC rules []. In this review, both names of heterocycle 1 are considered as synonyms.
Syntheses of derivatives of the heterocyclic system under discussion can be divided into two types depending on the starting synthons on which the tetracyclic framework is assembled. Synthetic approaches may involve a prefunctionalized benzimidazole on which a pyridopyrimidine scaffold is constructed (pathway 1) or a prefunctionalized pyrimidine ring on which a pyrido-benzimidazole scaffold is assembled (pathway 2).
The first derivatives of the heterocyclic heterocyclic system under discussion, named pyrimido[5’,4’:5,6] pyrido[1,2-a]benzimidazole 2, were obtained by reacting barbituric acid 5-carbaldehyde or 2,4,6-trichloropyrimidinyl-5-carbaldehyde with bis-(benzimidazol-2-yl)methane in N-methylpyrrolidone and are patented as photographic materials and luminescent dyes [-] (Scheme 1).
Scheme 1:
According to the other method, 1H-benzimidazole-2-acetonitrile (3) is condensed with arylidene malononitriles under the Michael reaction conditions, after which the adduct formed is boiled in MeCN in the presence of piperidine in a six-membered cycle with simultaneous aromatization. The thus formed 1-amino-3-arylpyrido[1,2-a]benzimidazole-2,4-dicarbonitriles 4 are converted by boiling with formamide to the desired 5-aryl-4-methylpyrimido[5’,4’:5,6 ]pyrido[1,2-a]- benzimidazole-6-carbonitrile 5, according to Scheme 2 [].
Scheme 2:
Dicarbonitriles 4 were the starting compounds also in the synthesis of the 3-amino-4-imino derivatives of the samе heterocyclic system according to scheme 3 [14].
Scheme 3:
In the synthesis of spirocondensed pyrimido[5’,4’:5,6] pyrido[1,2-a]benzimidazole derivatives, by reacting 2-methylbenzimidazole with 3-dicyanomethylidene-1-ethyl2-oxoindoline, cyano-3,4-dihydro-1’-ethylspiro {benzimidazo[1,2-a]pyridine-3,3’-indolin}-2’-one (7) were synthesized. The latter are cyclized by the action of formamide or formic acid to 4-amino-5,6-dihydro-1’-ethylspiro- {benzimidazo[1’,2’:1,6]pyrido[2,3-d]pyrimidine-5,3’- indoline}-2’-one and 3,5,6-trihydro-1’-ethylspiro{benzimi dazo[1’,2’:1,6]pyrido [2,3-d]pyrimidine-5,3’-indoline}-2’,4- dione . The latter was subsequently converted to 4-chloroand 4-hydrazino derivatives by the subsequent chlorination with POCl3 and hydrazinolysis according to Scheme 4 [15].
Scheme 4:
As follows from the above syntheses, in all developed approaches as the key compound is benzimidazole, in which the activated methylene and methyl groups and the HN group of benzimidazole act as a 1,3-binucleophilic center, due to which the cascade process of heterocyclization is launched. Based on this approach, relatively few series of compounds have been synthesized in which the range of functional substituents is limited to benzazoles, nitrile and amino groups, and aryl groups. Interestingly, the number of synthesized pyrimido[5’,4’:5,6]pyrido[1,2-a]benzimidazole derivatives is small, and there is no data on their biological activity. Limitations of approaches based on benzimidazoles are associated with the diffi culty of introducing a number of functional groups into the molecules, in particular, methylene and methyl groups in diff erent positions of the ring, aryl and sulfanyl groups in the pyrimidine fragment. Note that since the heterocyclic system of pyrimido[5’,4’:5,6] pyrido[1,2-a]benzimidazole is constructed by annulation of three π-deficient heterocycles, namely pyrimidine, pyridine and benzimidazole, it can also be classified as a π- deficient heterocycles. Taking this into account, the presence of activated methylene and methyl groups in π-deficient heterocyclic systems creates the possibility of obtaining new types of derivatives by condensation of these groups with carbonyl compounds to form compounds with extended π-conjugation chains. Moreover, the development of routes for the synthesis of derivatives of the discussed heterocyclic system, functionalized with reactive and pharmacophoric sulpha-, amino- and hydroxy groups, also seems promising. These requirements are met by the synthesis of pyrimido[5’,4’:5,6]pyrido[1,2-a]benzimidazole derivatives based on functionalized pyrimidinyl-5-propanoic acids.
In this regard, in recent years, a fundamentally new method for constructing the heterocyclic system under discussion has been developed based on readily available synthons - 2-substituted pyrimidinyl-5-propanoic acids, allowing methylene, methyl and sulfanyl groups to be introduced into the molecule according to Scheme 5.
Scheme 5:
It was found that the reaction of the corresponding 2-aryl-6-methyl(hydroxy)-3,4-dihydro-4-oxopyrimidine5-ylpropanoic and 2-methylpropanoic acids 9a-e with 1,2-diaminobenzene in polyphosphoric acid (PPA), of acids 9f,g - in a mixture of PPA-ZnCl2 proceeded by a cascade mechanism and led in a single step to a 4-methyl-, 4,6-dimethyl-4-hydroxy-6-methyl derivative of 2-aryl5,6-dihydro-benzo[4’,5’]imidazo[2’,1’:6,1]pyrido[2,3-d] pyrimidines 10a-e and the corresponding thiols 10f,g, and two disulfi des 11a,b. Disulfi des are formed in the form of an impurity with a yield of about 15% directly as a result of condensation, and also in the oxidation of 2-thioxoderivatives 10f,g with air oxygen. Oxidative aromatization of compound 10a with chloranyl was carried out to form a substituted benzo[4’,5’]imidazo[2’,1’:6,1]pyrido[2,3d]pyrimidine 14 with a 16π electron circuit [ 16-18]. Thiols 10f,g, in turn, are the starting compounds for further transformations. By oxidation with H2 O2 , a 2-hydroxy derivative 12a was obtained, which was chlorinated to a 2-chloro derivative 12b and put into an aminolysis reaction to obtain 2-amino derivatives 12c-e. By alkylation of tiols in an alkaline medium, aralkylsulfanyl derivatives 12f-i were obtained.
A very revealing comparison is the absorption bands in the IR spectra of the starting 2-phenyl-substituted pyrimidinyl-5-propanoic acid 9a and the corresponding tetracyclic product 10a. In the IR spectrum of acid 9a, absorption bands are observed in the form of protrusions at 3200 cm-1, 3140 cm-1 and 3070 cm-1, corresponding to the stretching vibrations of NH and OH groups, as well as several diffuse absorption bands in the region 3000 - 2500 cm -1, characteristic of stretching vibrations of carboxylic acid dimers. In addition, in the region of 1698 cm-1 and 1645 cm-1 there are strong bands of stretching vibrations of CO groups of the propanoic acid residue and the pyrimidine ring, respectively, and in the region of 1621 cm-1-1557 cm-1 there are C=C vibration bands and C=N bonds of the heterocycle. Substituted benzo[4’,5’]imidazo-[2’,1’,6,1]pyrido[2,3-d] pyrimidine 10a lacks all of the above absorption bands of NH-, OH- and CO-groups, with the exception of bands in the region of 1621 cm-1-1554 cm-1, caused by stretching vibrations of the C=C and C=N bonds of the heterocycle.
In the mass spectrum of tetracycle 10a, the maximum peak is the molecular ion m/z 312, and the intensity of the remaining peaks is much lower. Dissociative ionization in this case is accompanied by the initial release of the acetonitrile molecule due to the 4-CH3 group of the ring and recyclization into the azeto[3,2-e]benzo[4,5] aimidazo[1,2-a]pyridine derivative with the formation of a peak rearrangement ion m/z 270 of medium intensity (Scheme 6).
Scheme 6:
The X-ray structure of the 2-chloro-4-methyl-5,6 dihydrobenzo[4’,5’]imidazo[2’,1’:6,1]pyrido[2,3-d] pyrimidine (12b) derivative and its tetramer was obtained, presented below (Figure 1).
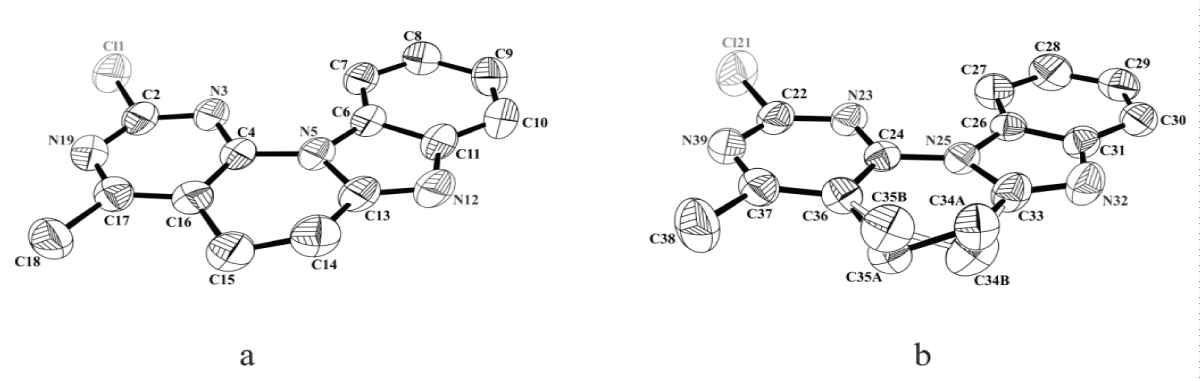 Figure 1: Structures of symmetrically nonequivalent molecules of compound 12b with ordered structure (a) and with disordered structure (b). Ellipsoids are depicted with 50% probability.
Figure 1: Structures of symmetrically nonequivalent molecules of compound 12b with ordered structure (a) and with disordered structure (b). Ellipsoids are depicted with 50% probability.X-ray diffraction analysis of the crystal of compound 12b showed that there are two symmetrically nonequivalent molecules in the unit cell. In this case, in one of the molecules there is a disordered structure associated with the statistical manifestation of two possible conformations of the piperidine ring (Figure 1b), while in the other molecule only one of the conformations appears (Figure 1a).
The molecule of the compound under study is tetracyclic. Considering the cyclic fragments separately, it was revealed that the phenyl, imidazole and pyrimidine rings have an almost planar conformation. The deviations of atoms from the average planes do not exceed 0.0055(5)Å, 0.0095(5)Å, 0.0117(5)Å, respectively. The piperidine ring has a half-chair conformation; the deviations of the C14 and C15 atoms from the half-chair plane are 0.2817(5)Å and -0.1633(5)Å, and the deviations of the C34(A,B) and C35(B,A) atoms from the corresponding plane _ by ±0.2526(5)Å and ±0.4100(5)Å. Analysis of the three-dimensional packing of molecules in the crystal lattice showed that molecules, connecting by non-classical hydrogen bonds (C14-H14B…..N39 and C15H15B…..N3i), form a tetramer (Figure 2). At the same time, the internal molecules of the tetramer have an ordered structure, and the final molecules have a disordered structure. In all likelihood, the conformations of the internal molecules are fixed by hydrogen bonds, due to which disorder is not observed for them (Figure 2).
Taking into account the fact that benzo[4’,5’]imidazo [2’,1’:6,1]pyrido[2,3-d]pyrimidine is a π-deficient heterocycle, the methyl and methylene groups of the heterocycle exhibit increased acidity and react with aromatic and heterocyclic aldehydes according to the Claisen condensation type with the formation of unsaturated compounds of two types 14, 15, depending on the carbonyl activity of the aromatic aldehyde (Scheme 7) [19,20]
It was shown that the reactive 4-methyl- and 6-methylene groups in the substituted 5,6-dihydro-benzo[4’,5’]imidazo[2’,1’:6,1]pyrido[2,3-d]pyrimidines reacted with aromatic aldehydes to form 6-aryl (heteryl)methyl-4-methyl derivatives 14a-g, and by co-heating in the presence of ZnCl2 - 4-substituted derivatives 15a,b and bis-substituted derivatives 16a-c.
Scheme 7:
The X-ray structure of the 6-benzyl derivative was obtained (Figures 3,4).
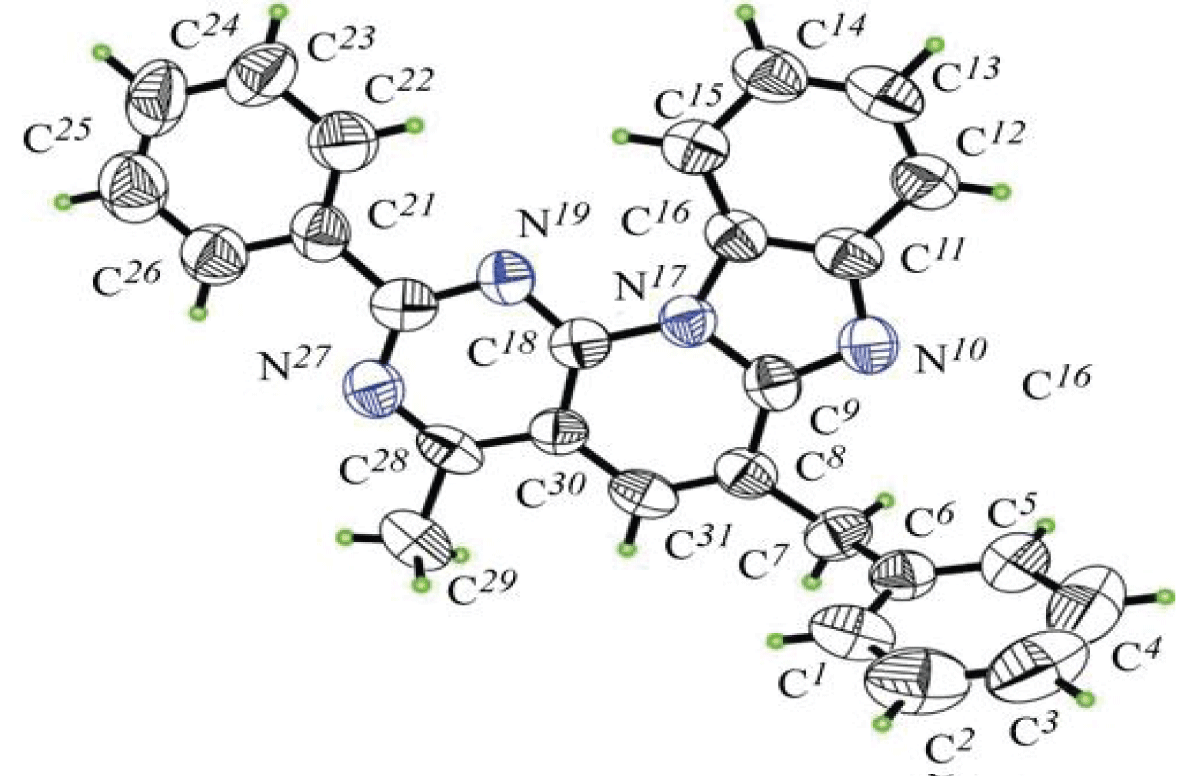 Figure 3: General view of the 6-benzyl-4-methyl-2-phenilbenzo[4’,5’]imidazo [2’,1’:6,1]pyrido[2,3-d]pyrimidyn (14a).
Figure 3: General view of the 6-benzyl-4-methyl-2-phenilbenzo[4’,5’]imidazo [2’,1’:6,1]pyrido[2,3-d]pyrimidyn (14a).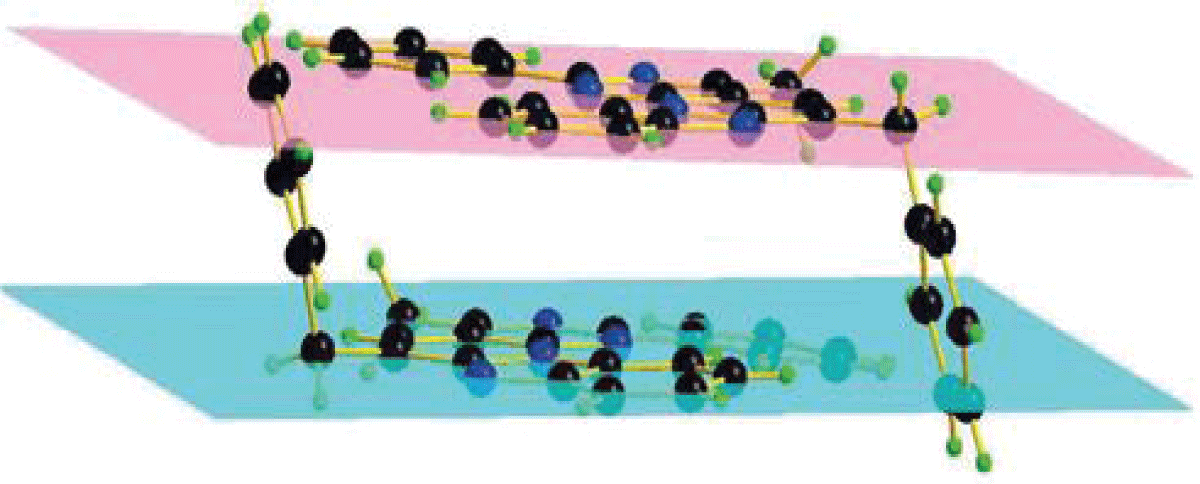 Figure 4: A pair of molecules 6-benzyl-4-methyl-2-phenylbenzo[4’,5’]imidazo[2’,1’:6,1]pyrido[2,3-d]pyrimidine (14a), linked interconnected by hydrophobic bonds.
Figure 4: A pair of molecules 6-benzyl-4-methyl-2-phenylbenzo[4’,5’]imidazo[2’,1’:6,1]pyrido[2,3-d]pyrimidine (14a), linked interconnected by hydrophobic bonds.X-ray diffraction analysis of the crystal of compound 14a showed that in there are 4 symmetrically nonequivalent molecules, and the total number of molecules in an elementary cell is equal to 32. Conformational calculations so far stated that all cyclic fragments have flat conformation, deviations of atoms from the normal the average planes do not exceed 0.0130(2) Å. In a three-dimensional packing of molecules, intermolecular interactions are mainly caused by forces van der Waals.
Based on the described methodology, a substituted bis-benzo[[4′,5′]imidazo-[2′,1′:6,1]pyrido[2,3-d]pyrimidine 17 was synthesized, in which two tetracyclic frameworks are connected via a triarylmethane linker [].
The developed strategy was successfully applied in the synthesis of a new pentacyclic heterocyclic system naphtho[1’’,2”:4,5]imidazo[2’,1’:6,1]pyrido[2,3-d] pyrimidine 18 by the reaction of pyrimidinyl-5- propanoic acid 9a with naphthalene-1,2-diamine in PPA (Scheme 8) [18].
Since in this case heterocyclization can lead to two different heterosystems 18 or 19, the structure of the resulting pentacycle as derivative of the naphtho[,,]imidazo[,,]-pyrido[,]pyrimidine 18 proven by NMR spectra and X-ray data.
The indicated structure of heterocycle 18 is supported by the presence of NОЕ between H13 and H2 in the 2D NOESY NMR spectrа (Scheme 8)(Figure 5).
Scheme 8:
The structure of the heterocycle was finally proven by X-ray diffraction (Figure 6).
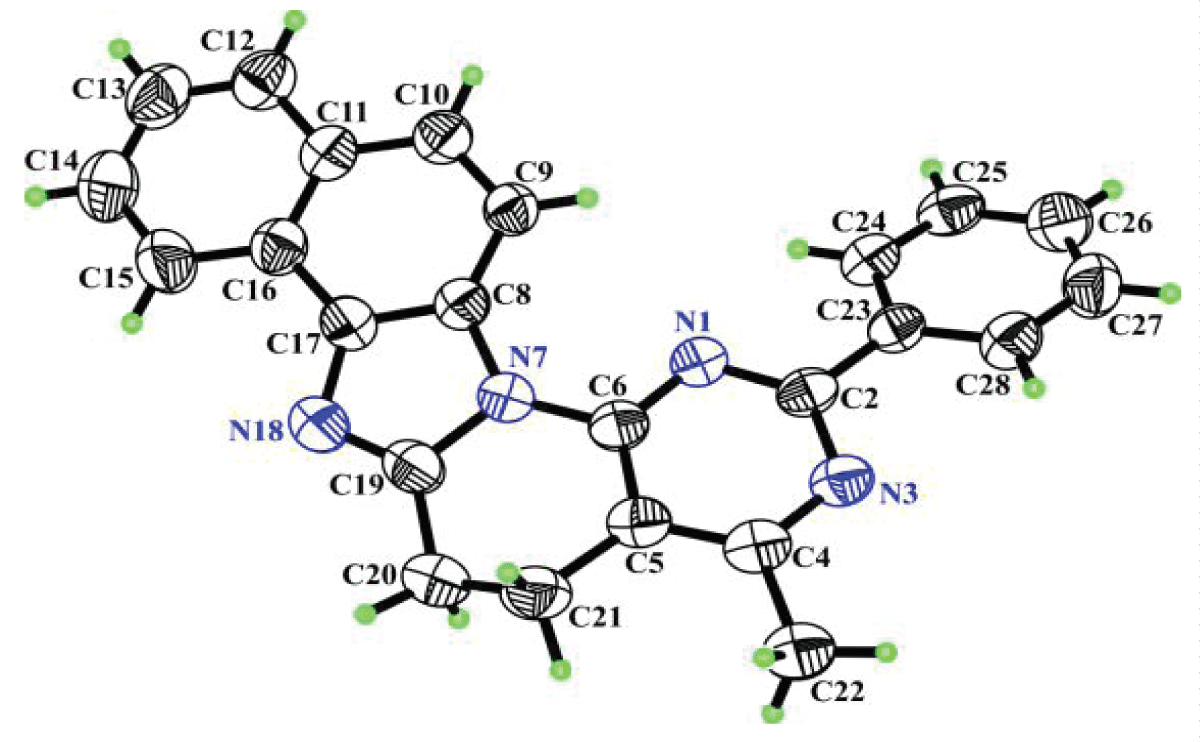 Figure 6: Molecule of compound 18 with arbitrary numbering of atoms. Ellipsoids of anisotropic thermal vibrations are depicted with 50% probability.
Figure 6: Molecule of compound 18 with arbitrary numbering of atoms. Ellipsoids of anisotropic thermal vibrations are depicted with 50% probability.Considering the conformation of the molecule, it turned out that the maximum deviation of atoms N1, C2, N3, C4, C5, C6 of the pyrimidine ring, atoms N7, C8, C17, N18, C19 of the naphtho-imidazole ring and atoms C23, C24, C25, C26, C27, C28 of the phenyl ring from the corresponding root-mean-square planes is less than 0.0137(28)Å, 0.0032(27)Å and 0.0140(28)Å, respectively. The above small deviations are a consequence of all the carbon atoms of these groups being in the sp2-hybridized state, and the deviation of the C20 and C21 atoms, which are in the sp3-hybridized state, from the plane of the C5, C6, N7 and C19 atoms is 0.3701(53)Å and -0.2611(49)Å. This deviation indicates a half-chair conformation for the piperidine fragment (C5, C6, N7, C19, C20, C21).
Data on the biological activity of benzo[4′,5′]imidazo[2’,1’:6,1]pyrido[2,3-d]pyrimidine derivatives have been published only in recent years [,].
Antibacterial properties of some derivatives of benzo[4′,5′]imidazo[2’,1’:6,1]pyrido-[2,3-d]pyrimidines have been studied for strains of gram-positive bacteria (Staphylococcus aureus 209p and S. aureus 1) and gram-negative rods (Shigella flexneri 6858, Escherichia coli 0-55) by the methods of “diff usion in agar” and “two-fold serial dilutions”, the control drug is furazolidone. It has been shown that benzo[4′,5′]imidazo[2’,1’: 6,1]pyrido[2,3-d] pyrimidines 11a, 12a and 12f with polar groups in position 2 the rings exhibit moderate antibacterial properties against all bacterial strains studied.
The antimonoaminoxidase properties of compounds were studied by their effect on the deamination of serotonin (5-OT) by the brain Monoamine Oxidase (MAO) in vitro, the control - drug indopan. It has been found that tetracycles 14d, 15b, and 16a show pronounced anti-MAO activity, inhibiting enzyme activity by 60% - 63%.
Research on benzo[4’,5’]imidazo[2’,1’:6,1]pyrido[2,3-d] pyrimidine derivatives at the current stage is associated with the construction of “pharmacological hybrids” based on them, that is, compounds the molecule of which combines the structures of heterocycles of various pharmacological profiles. This design of the molecule changes the affinity of the drug (ligand) to the target by expanding the possibilities of ligand-receptor interaction, which may be important in the creation of new, more selective and relatively low-toxic drugs. It is assumed that a planar fragment of a substituted benzo[4’,5’]imidazo[2’,1’:6,1]pyrido[2,3-d]pyrimidine, connected through a linker to another heterocycle with a different pharmacological action, can increase the affinity for nucleic acids and contribute to antitumor, antiviral and other types of activity due to the mechanism of intercalation between nucleic acid bases. We have synthesized a number of hybrid molecules with the general design presented below, which are at the stage of docking studies and preliminary biological studies.
It was found that some compounds of the “hybrid” type in vitro on the sarcoma 180 (C-180) model exhibit a high level of suppression of DNA methylation, equal to or significantly higher than that for the antibiotic doxorubicin. The same data were confirmed in docking studies conducted on the complex of RNA-dependent RNA polymerase (RdRp) with the proteins NSP7 and NSP8 of the SARS-CoV-2 virus, the kinase domain of the human epidermal growth factor receptor (EGFR) and double phosphorylated human mitogen-activated protein kinase 14 (MAPK14) in complex with activating transcription factor (ATF2) [].
Jampilek J. Heterocycles in Medicinal Chemistry. Molecules. 2019 Oct 25;24(21):3839. doi: 10.3390/molecules24213839. PMID: 31731387; PMCID: PMC6864827.
Sharma PK, Amin A, Kumar M. A Review: Medicinally important nitrogen sulphur containing heterocycles. Open Med. Chem. J. 2020; 14:49-64.org/10.2174/1874104502014010049
Kabir E, Uzzaman M. A review on biological and medicinal impact of heterocyclic compounds. Results in Chemistry. 2022; 4:100606. doi.org/10.1016/j.rechem.2022.100606
Sharma V, Gupta M, Kumar P, Sharma A. A Comprehensive Review on Fused Heterocyclic as DNA Intercalators: Promising Anticancer Agents. Curr Pharm Des. 2021;27(1):15-42. doi: 10.2174/1381612826666201118113311. PMID: 33213325.
Jubete G, de la Bellacasa PR, Estrada-Tejedor R, Teixido J, Borrell JI. Pyrido[2,3-d]pyrimidin-7(8H)-ones: synthesis and biomedical applications. Molecules. 2019; 24:4161. doi.org/10.3390/molecules24224161
Tahlan S, Kumar S, Narasimhan B. Pharmacological significance of heterocyclic 1H-benzimidazole scaffolds: a review. BMC Chem. 2019 Aug 6;13(1):101. doi: 10.1186/s13065-019-0625-4. PMID: 31410412; PMCID: PMC6685272.
Xiong H, Yu Q, Ma H, Yu X, Ouyang Y, Zhang ZM, Zhou W, Zhang Z, Cai Q. Exploration of tricyclic heterocycles as core structures for RIOK2 inhibitors. RSC Med Chem. 2023 Jul 21;14(10):2007-2011. doi: 10.1039/d3md00209h. PMID: 37859717; PMCID: PMC10583808.
CA Chem. Substance Index. v.95, 6179CS. 1981.
Hunter AD. ACD/ChemSketch 1.0 (freeware); ACD/ChemSketch 2.0 and its tautomers,dictionary, and 3D plug-ins; ACD/HNMR 2.0; ACD/CNMR 2.0. Chem. Educ. Easton.1997;74(8):905-908.
Pyrimido[5’,4’:5,6]pyrido[1,2-a] Ger. Offen. Chem. Abstr. 1981; 95: P44732; 2.929. 414.
Electrophotographic recording material. Wiedemann W. (Hoechst A.-G.). Ger. Offen. DE3.502.689, 33 pp. Chem. Abstr. 1987; 106:41561j.
Electrophotographic recording material. Wiedemann W, Guenther D. (Hoechst A.-G.). Ger.Offen. Chem. Abstr. 1988; 108: DE 3.502.681;28:122003x.
Bogdanowicz-Szwed K, Czarny A. Synthesis of polyazaheterocycles by Michael addition of CH acids to a α,β-unsaturated nitriles. Synthesis of pyrido[1,2-a]benzimidazole and pyrimido[5′,4' : 5,6]pyrido[l,2-a]benzimidazole derivatives. J. prakt. Chem. 1993; 335:279. org/10.1002/prac.19933350311
Elwan NM. First synthesis of novel pentaheterocyclic ring system of 1,2,4-triazolo[2",3":6',1']pyrimido[4',5':2,3]pyrido[1,2-a] J. Heterocyclic Chem. 2004; 41:281. doi.org/10.1002/jhet.5570410222
El-Zohry MF, Mohamed TA, Hussein EM. Novel syntheses of some new 3,4- dihydrospiro{benzimidazo[1,2-a]pyridine-3,30-indolin}-20-one derivatives. Monatsh. Chem. 2009; 140:265. org/10.1007/s00706-008-0013-6
Harutyunyan AA. Synthesis of the derivatives of the new heterocyclic system 5,6-dihydrobenzo[4',5']imidazo[2',1':6,1]pyrido[2,3-d] Chem. J. Armenia. 2012; 65(2):257. https://arar.sci.am/publication/204311
Harutyunyan АA, Panosyan HA, Tamazyan RA, Ayvazyan AG. New derivatives of the benzo[4,5]imidazo[2',1':6,1]pyrido[2,3-d] Chem. J. Armenia. 2016; 69(3):266. https://arar.sci.am/publication/205621
Harutyunyan AA. Studies in the field of the pyrimidines and polycyclic azaheterocycles synthesis. The dissertation abstract of the doctor of chemical sciences. Armenia, Yerevan. 2017.
Harutyunyan AA. Syntheses and biological properties of benzo[4', 5']imidazo[2',1': 6,1]-pyrido[2,3-d]pyrimidines: mini-review. Chem. J. Armenia. 2018; 71(4):579. https://arar.sci.am/publication/206351
Harutyunyan AA, Gukasyan GT, Panosyan HA, Tamazyan RA, Ayvazyan AG, Grigoryan AG, Danagulyan GG. Synthesis of new derivatives of benzo[4',5']imidazo[2 ',1': 6,1]pyrido[2,3-d] Russ. J. Org. Chem. 2019; 55(11):1698. doi.org/10.1134/S1070428019110095
Harutyunyan AA, Israelyan SG, Safaryan MS, Hakobyan MR, Sumbatyan AS, Dilanyan SV. Synthesis and biological properties of derivatives of five- and six-membered azaheterocycles and their condensed systems. Brief review of research by the laboratory for the synthesis of anti-cancer compounds in 2019-2023. Some Successes Organic and Pharmaceutical Chemistry. Collection of works. Issue 4, p. 112-135 (in English). Armenia, Yerevan. 2023; ISBN 978-9939-1-1712-6.
Harutyunyan A. Benzo[4’,5’]imidazo[2’,1’:6,1]pyrido[2,3-d]pyrimidines: Past and Present. IgMin Res. Nov 16, 2023; 1(1): 025-031. IgMin ID: igmin113; DOI: 10.61927/igmin113; Available at: www.igminresearch.com/articles/pdf/igmin113.pdf
次のリンクを共有した人は、このコンテンツを読むことができます:
Address Correspondence:
AA Harutyunyan, The Scientific Technological Centre of Organic and Pharmaceutical Chemistry of NAS RA 26, Azatutyan Ave., Yerevan, 0014, Armenia, Email: [email protected]
How to cite this article:
Harutyunyan A. Benzo[4’,5’]imidazo[2’,1’:6,1]pyrido[2,3-d]pyrimidines: Past and Present. IgMin Res. Nov 16, 2023; 1(1): 025-031. IgMin ID: igmin113; DOI: 10.61927/igmin113; Available at: www.igminresearch.com/articles/pdf/igmin113.pdf
Copyright: © 2023 Harutyunyan AA. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
 Figure 2: Tetramer of molecules of compound 12b formed using...
Figure 2: Tetramer of molecules of compound 12b formed using...
![General view of the 6-benzyl-4-methyl-2-phenilbenzo[4’,5’]imidazo [2’,1’:6,1]pyrido[2,3-d]pyrimidyn (14a).](https://www.igminresearch.jp/articles/figures/igmin113/igmin113.g003.png) Figure 3: General view of the 6-benzyl-4-methyl-2-phenilbenz...
Figure 3: General view of the 6-benzyl-4-methyl-2-phenilbenz...
![A pair of molecules 6-benzyl-4-methyl-2-phenylbenzo[4’,5’]imidazo[2’,1’:6,1]pyrido[2,3-d]pyrimidine (14a), linked interconnected by hydrophobic bonds.](https://www.igminresearch.jp/articles/figures/igmin113/igmin113.g004.png) Figure 4: A pair of molecules 6-benzyl-4-methyl-2-phenylbenz...
Figure 4: A pair of molecules 6-benzyl-4-methyl-2-phenylbenz...
 Figure 5: 2D NOESY NMR spectrum of compound 18....
Figure 5: 2D NOESY NMR spectrum of compound 18....
 Figure 6: Molecule of compound 18 with arbitrary numbering o...
Figure 6: Molecule of compound 18 with arbitrary numbering o...
 Figure i1: ...
Figure i1: ...
 Figure i2: ...
Figure i2: ...
 Figure i3: ...
Figure i3: ...
 Figure s1: ...
Figure s1: ...
 Figure s2: ...
Figure s2: ...
 Figure s3: ...
Figure s3: ...
 Figure s4: ...
Figure s4: ...
 Figure s5: ...
Figure s5: ...
 Figure s6: ...
Figure s6: ...
 Figure s7: ...
Figure s7: ...
 Figure s8: ...
Figure s8: ...
Jampilek J. Heterocycles in Medicinal Chemistry. Molecules. 2019 Oct 25;24(21):3839. doi: 10.3390/molecules24213839. PMID: 31731387; PMCID: PMC6864827.
Sharma PK, Amin A, Kumar M. A Review: Medicinally important nitrogen sulphur containing heterocycles. Open Med. Chem. J. 2020; 14:49-64.org/10.2174/1874104502014010049
Kabir E, Uzzaman M. A review on biological and medicinal impact of heterocyclic compounds. Results in Chemistry. 2022; 4:100606. doi.org/10.1016/j.rechem.2022.100606
Sharma V, Gupta M, Kumar P, Sharma A. A Comprehensive Review on Fused Heterocyclic as DNA Intercalators: Promising Anticancer Agents. Curr Pharm Des. 2021;27(1):15-42. doi: 10.2174/1381612826666201118113311. PMID: 33213325.
Jubete G, de la Bellacasa PR, Estrada-Tejedor R, Teixido J, Borrell JI. Pyrido[2,3-d]pyrimidin-7(8H)-ones: synthesis and biomedical applications. Molecules. 2019; 24:4161. doi.org/10.3390/molecules24224161
Tahlan S, Kumar S, Narasimhan B. Pharmacological significance of heterocyclic 1H-benzimidazole scaffolds: a review. BMC Chem. 2019 Aug 6;13(1):101. doi: 10.1186/s13065-019-0625-4. PMID: 31410412; PMCID: PMC6685272.
Xiong H, Yu Q, Ma H, Yu X, Ouyang Y, Zhang ZM, Zhou W, Zhang Z, Cai Q. Exploration of tricyclic heterocycles as core structures for RIOK2 inhibitors. RSC Med Chem. 2023 Jul 21;14(10):2007-2011. doi: 10.1039/d3md00209h. PMID: 37859717; PMCID: PMC10583808.
CA Chem. Substance Index. v.95, 6179CS. 1981.
Hunter AD. ACD/ChemSketch 1.0 (freeware); ACD/ChemSketch 2.0 and its tautomers,dictionary, and 3D plug-ins; ACD/HNMR 2.0; ACD/CNMR 2.0. Chem. Educ. Easton.1997;74(8):905-908.
Pyrimido[5’,4’:5,6]pyrido[1,2-a] Ger. Offen. Chem. Abstr. 1981; 95: P44732; 2.929. 414.
Electrophotographic recording material. Wiedemann W. (Hoechst A.-G.). Ger. Offen. DE3.502.689, 33 pp. Chem. Abstr. 1987; 106:41561j.
Electrophotographic recording material. Wiedemann W, Guenther D. (Hoechst A.-G.). Ger.Offen. Chem. Abstr. 1988; 108: DE 3.502.681;28:122003x.
Bogdanowicz-Szwed K, Czarny A. Synthesis of polyazaheterocycles by Michael addition of CH acids to a α,β-unsaturated nitriles. Synthesis of pyrido[1,2-a]benzimidazole and pyrimido[5′,4' : 5,6]pyrido[l,2-a]benzimidazole derivatives. J. prakt. Chem. 1993; 335:279. org/10.1002/prac.19933350311
Elwan NM. First synthesis of novel pentaheterocyclic ring system of 1,2,4-triazolo[2",3":6',1']pyrimido[4',5':2,3]pyrido[1,2-a] J. Heterocyclic Chem. 2004; 41:281. doi.org/10.1002/jhet.5570410222
El-Zohry MF, Mohamed TA, Hussein EM. Novel syntheses of some new 3,4- dihydrospiro{benzimidazo[1,2-a]pyridine-3,30-indolin}-20-one derivatives. Monatsh. Chem. 2009; 140:265. org/10.1007/s00706-008-0013-6
Harutyunyan AA. Synthesis of the derivatives of the new heterocyclic system 5,6-dihydrobenzo[4',5']imidazo[2',1':6,1]pyrido[2,3-d] Chem. J. Armenia. 2012; 65(2):257. https://arar.sci.am/publication/204311
Harutyunyan АA, Panosyan HA, Tamazyan RA, Ayvazyan AG. New derivatives of the benzo[4,5]imidazo[2',1':6,1]pyrido[2,3-d] Chem. J. Armenia. 2016; 69(3):266. https://arar.sci.am/publication/205621
Harutyunyan AA. Studies in the field of the pyrimidines and polycyclic azaheterocycles synthesis. The dissertation abstract of the doctor of chemical sciences. Armenia, Yerevan. 2017.
Harutyunyan AA. Syntheses and biological properties of benzo[4', 5']imidazo[2',1': 6,1]-pyrido[2,3-d]pyrimidines: mini-review. Chem. J. Armenia. 2018; 71(4):579. https://arar.sci.am/publication/206351
Harutyunyan AA, Gukasyan GT, Panosyan HA, Tamazyan RA, Ayvazyan AG, Grigoryan AG, Danagulyan GG. Synthesis of new derivatives of benzo[4',5']imidazo[2 ',1': 6,1]pyrido[2,3-d] Russ. J. Org. Chem. 2019; 55(11):1698. doi.org/10.1134/S1070428019110095
Harutyunyan AA, Israelyan SG, Safaryan MS, Hakobyan MR, Sumbatyan AS, Dilanyan SV. Synthesis and biological properties of derivatives of five- and six-membered azaheterocycles and their condensed systems. Brief review of research by the laboratory for the synthesis of anti-cancer compounds in 2019-2023. Some Successes Organic and Pharmaceutical Chemistry. Collection of works. Issue 4, p. 112-135 (in English). Armenia, Yerevan. 2023; ISBN 978-9939-1-1712-6.